Endocrine Disruption and Perspective Human Health Implications: A Review
S Poongothai, R Ravikrishnan, P Murthy
Keywords
developmental exposure, endocrine disrupting compounds edcs, hormones, neurotoxicity, pesticides, reproductive function
Citation
S Poongothai, R Ravikrishnan, P Murthy. Endocrine Disruption and Perspective Human Health Implications: A Review. The Internet Journal of Toxicology. 2007 Volume 4 Number 2.
Abstract
Over the last decades, many tone of man - made chemicals have been produced and released into the environment. Many of these environmental contaminants have the ability to modulate the action of hormones by interacting with hormone receptors and mimic or antagonize the production and activities of endogenous hormones are called endocrine disruptors. The public health implications of these so called endocrine disruptors have been the subject of scientific debate, media interest and policy attention over the past several years. In this article we review the background of environmental endocrine disruption, developmental effects due to early exposure of Endocrine disrupting compounds (EDCs), assessing mode of action of endocrine disruptors, disruption of female hormonal function and its potential effects, and current evidence of possible impacts on reproduction system and neurobehavioral function. Finally, worldwide health policy activities that are relevant to endocrine-disrupting compounds in the environment are discussed.
Introduction
Current widespread interest in a range of health effects potentially associated with endocrine-disrupting compounds (EDCs) has made exposure assessment for these compounds a priority. Studies of potential health effects associated with EDCs have been hampered by lack of information about the major sources of exposure to EDCs. Furthermore, because many EDCs act additively through a common mechanism of action or have antagonistic or other interactive effects by operating at different points in cell signaling systems, consideration of exposure to mixtures is critical in studies of health effects. Hormones play a crucial role in guiding normal cell differentiation in early life forms, and so exposure to endocrine disrupting substances in the egg or in the womb can alter the normal process of development. Mature animals can also be affected, but in the developing organism it is especially vulnerable. Exposure at this sensitive time may cause effects that are not evident until later in life, such as effects on learning ability, behavior, reproduction and increased susceptibility to cancer and other diseases. Many pesticides are now suspected of being endocrine disruptors-compounds that can lead to an increase in birth defects, sexual abnormalities and reproductive failures 1 . Specific effects found in wildlife and laboratory studies on mammals, reptiles, amphibians and fish include abnormal blood hormone levels, reduced fertility, altered sexual behavior, modified immune system, masculinization of females, feminization of males, undescended testicles, reduced penis size and testis, altered bone density and structure, cancers of the male and female reproductive tract, and lastly, malformed fallopian tubes, uterus and cervix. Other than pesticides some of the industrial chemicals also cause endocrine disruption like phthalates, a class of chemicals used as plasticizers, have attracted special attention because of their high-volume production, environmental presence and possible association with adverse reproductive health outcomes in females 23 . According to recent biomonitoring studies exposure of the general population to phthalates is widespread 45 .
Wildlife will be especially vulnerable to the endocrine disrupting effects of pesticides, because these chemicals are deliberately released into the environment. Humans exposed occupationally are at increased risk, and there are studies linking exposure to endocrine-disrupting compounds which comprise a diverse group of compounds and their observed health effects include estrogenic, androgenic, anti-estrogenic and antiandrogenic effects and disruption of thyroid function, the immune system, sexual differentiation of the brain during fetal development and motor function. EDCs may also have carcinogenic effects 6 and significant decrease of testis weight, epididymal sperm counts, sperm motility and marker testicular enzymes for testosterone biosynthesis 7 . The developing offspring is the most sensitive target of endocrine disruption. Many man-made chemicals can cross the placental barrier, thereby allowing the mother's body burden to be shared with her developing offspring. Further, intake occurs as nursing animals drink fat-loving chemicals that are bound into fat-rich milk. In egg laying species, the chemicals are transported from mother to the egg yolk where the chemicals cause irreversible damage during incubation. These concentrated doses of chemicals during embryonic, fetal and early postnatal development can be the highest exposures encountered throughout life. Again, the timing for such exposures is of concern since much of the neural, reproductive, and immune development continues long after birth or hatching 8 .
Fetal liability and developmental exposure
In a recent USEPA summary report 9 , defined liability applied to risk assessment as a four-component system: (1) susceptibility or sensitivity of the human or ecological receptors; (2) differential exposures of the receptors; (3) differential preparedness of the receptor to withstand the insult from exposure; (4) differential ability to recover from these effects. All of these components are pertinent to systems undergoing development from the fetus through childhood. The developing child (fetus to prepuberty) is particularly susceptible to environmental insult, because development is a highly integrated process in which high rates of proliferation and extensive differentiation are coordinated with each other and with programmed cell death. Rapid growth rates allow for mutagenic and epigenetic alterations as cells proliferate. Some pesticides and other industrial chemicals can disturb development of the endocrine system and of the organs that respond to endocrine signals in organisms indirectly exposed during prenatal and/or early postnatal life;
For many years now, there has been public concern raised about the potential health effects of pesticides on the developing fetus and in childhood. Depending on stage of development, the fetus is selectively sensitive to particular chemical toxicants. There is an increasing concern for adverse health outcomes after developmental early life exposure to environmental compounds that trouble the endocrine system is explained in Fig. 1. Gestational and prenatal exposures to EDCs may have long-term effects on the endocrine system that can influence tumor development later in life. Alexander et al., 14 recently demonstrated that
Assessing mode of action and potential health implications
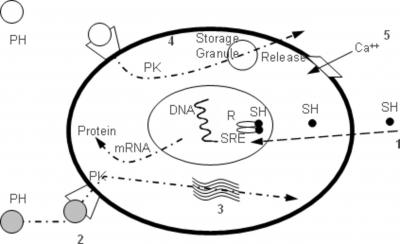
Information regarding mode of action of endocrine disruptor improves the scientific basis for risk decisions. The processes involved in the mode of endocrine disruptors are much more complicated with the interconnection of nervous, endocrine and immune systems. Endocrine-disrupting compounds are known to act at multiple sites through multiple modes of action, but the mechanism of action including putative exposure-response relationships are poorly understood 19 . It is necessary to explore the mechanisms of action from multiple points of view. Numerous substances, such as dioxins, polychlorobiphenyls (PCBs), DDT (dichlorodiphenyltrichloroethane) and phthalates have been suspected of working through endocrine-disruptive mechanisms 20 . Environmental agents can alter endocrine function through a variety of mechanisms and some of the better-studied cellular target sites are included in Fig. 2.
Figure 2
Some of the better-studied cellular target sites include (1) steroid hormone receptor-mediated changes in protein synthesis and/or mitosis, alterations that occur after exposure to (2) compounds that interfere with membrane receptor binding, (3) steroidogenesis, (4) compounds that interfere with the synthesis of other hormones and (5) compounds that alter the flux of ions across the membrane within certain types of hormone-secreting cells. Ca ++ =calcium ion, SH=steroid hormone in blood or cytoplasm, PH=peptide hormone, R=steroid hormone receptor, SRE=steroid response element, PK=protein kinase.
Many pesticides have now been found to have estrogenic or anti-androgenic activity, and some bind to the androgen or estrogen receptors. Those which have been found to bind to the estrogen receptor include: ortho-phenylphenol 1, DDT and metabolites (although the anti-androgenic properties of p'p' DDE may be of greater importance); methoxychlor; chlordecone 2, dieldrin, endosulfan; 1-hydroxychlordene (a metabolite of chlordane), and toxaphene 21 . In recent years, there has been growing concern over the possibility of hormonal disruption at low levels of exposure. The traditional risk assessments of environmental contaminants are based on the extrapolation of high-dose data in experimental systems to estimate the effects at much lower doses in humans. However, the validity of such extrapolations is frequently challenged if one considers that low levels of exposure. Some of these can induce estrogenic effects at relatively low levels. For example, administration of methoxychlor to the new born rat at a dose level of 0.5 µg per day caused accelerated puberty and accelerated loss of fertility. The dose levels at which effects are noted are fairly low 22 .
Ahmed 23 has reported extensive study into the effects of pesticides on immune system functioning; they noted that several pesticides which are commonly used in residential and agricultural areas may suppress normal human immune responses. It was found that endocrine disruptors are not merely affects the ‘reproductive hormones', but also affect the functioning of several non reproductive tissues, notably the immune system. Pyrethroids can elicit a range of immunotoxic and neurotoxic effects in humans and other mammals, and their exposure may contribute to reproductive dysfunction, developmental impairment and cancer 24 . Organophosphates can interfere with the immune system and exert immunotoxic effects directly or indirectly. Immunotoxicities are varied and include pathology of immune organs, and decreased humoral and/or cell mediated immunity, decreased non-specific immunity, decreased host resistance, hypersensitivity and autoimmunity 25 . Permethrin and cypermethrin showed dose-dependent cytotoxicity. The most toxic pyrethroid was cypermethrin followed by permethrin and natural pyrethrin. This study confirms that the cell toxicity was dependent on the chemical structure and pyrethroids without an ?-cyano group shows the weakest physiological effect 26 . Treatment with cypermethrin caused significant decreases in ejaculate volume, sperm concentration, total sperm output, sperm motility and plasma testosterone 27 . Synthetic halogenated chemicals increase liver tumors after early life-stage exposure. Recently, a prototypical endocrine-disrupting compound, 2, 3, 7, 8 - tetrachlorodibenzo-p-dioxin (TCDD), has been shown to be a developmental toxicant of the mammary gland in rodents 28 . No significant changes were detected in nipple development and anogenital distance of female offspring at any dose level tested for EDC. In contrast to males, sexual differentiation of females is largely hormone-independent, yet still susceptible to hormonal disruption, e.g., masculinisation of the female fetus by exposure to androgens 29 .
Disruption of female hormonal function
Study on endocrine disruption is an emerging field; researches are needed to give deep perception in endocrine disruptors. Immature organisms are especially susceptible to endocrine disruption because the reproductive system is under development and relatively small changes in endogenous hormone levels may result in permanent structural and functional changes 30 .The development and the function of female reproductive tract depend upon hormone concentrations and balance. The endocrine disruptors modulate the hormonal function in the body and, in particular, affect the steroid hormones. Changes in effective concentrations of hormones can occur if an endocrine disruptor binds to a specific hormone receptor. This chemical substance may then either mimic the hormone or block the normal biological response by occupying the receptor site. Alternatively, endocrine disruptors may able to react directly or indirectly with the hormone structure to alter its function, modulate the number of hormone receptor, change the way of hormone synthesis and their affinities for specific molecules.
Interference with hormone synthesis¡
Some EDCs, such as fenarimol, prochloraz, and other imidazole fungicides possess the ability to inhibit estrogen biosynthesis through CYP19 aromatase inhibition in vitro, preventing the conversion of androgens to estrogens. Vinggaard et al., 31 hypothesized that compounds which can inhibit aromatase activity in vitro may be able to cause local changes in estrogen and androgen concentrations
Interference with hormone storage and release
Catecholamine hormones (e.g. norepinephrine) are stored in granular vesicles of chromomaffin cells within the adrenal medulla and within presynaptic terminals in the central nervous system. Therefore, they can be released quickly on demand. In contrast, steroid hormones are not stored intracellularly within secretory granules, but are readily synthesized after gonadotropin stimulation of the gonads. Norepinephrine is critical for the preovulatory increase in the pulsatile release of GnRH (gonadotropin releasing hormone) and the subsequent ovulatory surge of LH. Fenvalerate directly or indirectly, or secondarily alter Ca 2+ homeostasis in ovarian cells, it may impair the reproductive endocrine functions. Furthermore, accumulating evidences indicate calcium ions modulate gonadotropin-stimulated steroidogenesis by granulosa cells 33 .
Interference with hormone transport and clearance
Steroid hormones in the bloodstream do not float around freely, but are bound to carrier proteins, such as SHBG (Sex-Hormone Binding Globulin) and albumin. Because only free hormones can be biologically active, increases or decreases in the concentration of SHBG will have a major impact on the available and active steroid hormone concentrations in blood. Estrogens are known to increase the synthesis of SHBG in the liver and thus increase the SHBG concentration in plasma, whereas androgens decrease these concentrations. Generally, clearance occurring in liver and the clearance rate is different for each hormone and is influenced by compounds that alter liver enzyme activity involved in hormone clearance. Many pesticides induce the liver enzymes monooxygenase and UDP-glucuronosyltransferase, resulting in increased clearance of the pesticide itself for detoxification purposes. Marty et al., 34 demonstrated a weak response following exposure to 50 and 100 mg/kg body weight of phenobarbital, with a decrease in T4 but no effect on TSH or thyroid histology. They recently showed that thyrotoxic effects can also be observed following exposure to toxicants which act via induction of liver enzymes and subsequent clearance of T4 35 . In this study, T4 concentration was the most sensitive endpoint, with TSH and thyroid histology having lower sensitivity.
Interference with hormone receptor and binding
Hormones travel from their point of release in the bloodstream to particular tissues where they convey their messages. For the message to be interpreted, hormones bind to receptors. Hormone and receptor have a precise fit, so that only a specific type of hormone can bind to a specific receptor. A number of environmental agents may alter this process by mimicking the natural hormone (agonists) or by inhibiting receptor binding (antagonists). The latter mechanism is based on complete or partial blocking of the specific receptor. Regarding the estrogen receptor, this mechanism only applies when the endocrine disruptor concentration is high, because the affinity of endocrine disruptors for the estrogen receptor is usually many times lower than that of 17-?-estradiol. Three different mechanisms are involved usually during interference with hormone receptor by EDCs which includes (1) binding and activating the estrogen receptor; (2) binding without activating the estrogen receptor; and (3) binding with other receptors 36 . For example pyrethroid compounds (permethrin, cypermethrin, fenvalerate and deltamethrin) at high concentrations inhibited the binding of estradiol to rat uterus cystolic estrogenic receptors (ERs) in a competitive binding study and suggested that signaling pathways other than ERs are involved in mediating pyrethroid induced or inhibited MCF-7 cell proliferation and pyrethroid insecticides may alter the conformation ER without binding to it.
Interference with the thyroid function
Normal thyroid hormone blood levels are essential for growth and development of tissues and for the maintenance of tissue and organ function. Large numbers of environmental chemicals have been reported to affect production, transport, or metabolism of thyroid hormones, some acting by more than one mechanism 37 . Zaidi et al., 38 found that TSH was elevated, total T3 suppressed, and T4 marginally decreased in pesticide formulators. Long term exposure to Ammonium perchlorate which is an inorganic ion used as a primary ingredient in rocket fuel, paints, fertilizers and lubricants which has recently been detected in drinking water supplies in the United States 39 , the thyroid axis is significantly altered as evidenced by decreased thyroxin in serum and a subsequent rise in TSH.
Interference with the central nervous system
The central nervous system (CNS) is very important in the integration of hormonal and behavioral activity. Disturbances in these finely tuned mechanisms can severely impair normal adaptive behavior and reproduction. Since many pesticides are known to be neurotoxic, it is conceivable that these compounds can disrupt the coordinating activity of the CNS by disrupting brain cell functions. Also, pesticides can alter the hypothalamic and pituitary function and thus secretion of GnRH, LH, and FSH in a more direct manner by modifying the feedback of endogenous hormones. In relation to the early development of the female CNS and reproductive systems the more accepted theory is that which occurs in the absence of any significant gonadal hormone actions. However, female mice knockout of an aromatase gene (ArKO) (deficient in aromatase activity) and therefore unable to aromatize androgen to estrogen, suggesting that estradiol is required for the development of the neural mechanisms controlling this behavior in mice 40 .
Potential effects on female hormone disruption
Large numbers of environmental chemicals have been reported to affect production, transport, or metabolism of hormones, some acting by more than one mechanism. Endocrine disruption may result in disturbances in the reproductive system, such as modulation of hormone concentrations, ovarian cycle irregularities, and impaired fertility. The organochlorine pesticide hexachlorobenzene (HCB) is a worldwide persistent organic pollutant and has been detected in various tissues and human fluids including serum and ovarian follicular fluid. Any environmental compound mimicking or antagonizing steroid hormone action could presumably alter the glycosylation of LH and FSH, thereby reducing their biological activity 41 . Endocrine disruptors with estrogenic properties may be able to block ovulation similar to contraceptive pills. Human fertility is a delicate process that can be influenced by many factors, such as hormonal imbalance caused by pesticides. However, in most studies it is not clear whether impaired fertility is due to hormonal imbalance or to other toxic effects. The pubertal female rat assay is designed to detect alterations in thyroid hormone status, hypothalamic–pituitary–gonadal (HPG) function, inhibition of steroidogenesis ERs and antiestrogens 42 .
Reproduction and neurobehavioral effects
Endocrine-disrupting compounds are among the most complex environmental health threats known today. Reproductive toxicity begins with parental exposure to toxicants. Preconception, conception, prenatal, and postnatal periods are all windows of opportunity for adverse reproductive outcomes. By mimicking natural hormones such as estrogen and testosterone, these chemicals can interact with the body's endocrine system and exert toxic effects that may lead to reproductive and developmental abnormalities or cancer. The reproductive hazards of a chemical can affect men, women, the fetus and postnatal development. Moniz et al., 43 reported that there was a delay in vaginal opening, suggested that perinatal fenvalerate exposure may induce alterations on sexual maturation in females and the only external signal of puberty is canalization of the vagina, which is initially imperforated, and later becomes patent as a consequence of estrogenic stimulation. Transgenerational effects on reproductive capacity may be a common feature of endocrine-disrupting chemicals. Pesticides may cause hormonal imbalance and reproductive toxicity, especially in female through several different mechanisms: direct damage to the structure of cells, interference with biochemical processes necessary for normal cell function, and biotransformation resulting in toxic metabolites (Fig. 3). Anway et al., 44 reported very recently that pregnant rats treated with the estrogenic pesticide methoxychlor or the anti-androgenic fungicide vinclozolin have subfertile offspring, and that the male offspring pass the defect through the male germ line for at least four generations. They also reported that there is an altered pattern of DNA methylation in the male germ line. Developmental treatment with Diethylstilbestrol (DES) caused an alteration in gene imprinting as demonstrated by the penetrance of a tumor suppressor gene in the uterus. Thus, in several model systems gene imprinting by estrogen is associated with later uterine disease. The heritability of these changes can be seen by the transmission of disease to the next generation. Mice exposed to DES early in development, when mated to control males, produced offspring that also had increased risk for vaginal adenocarcinomas. Thus, the ‘granddaughters' of the treated mice expressed the same rare cancer as the daughters. The implications for transmission of epigenetic traits are obvious, as is the possibility for transgenerational transmission of the DES effect in humans 45 . Since 1997 a large number of peer reviewed journal articles has been showing that bisphenol-A harm in animals at levels to which the average human is exposed. Bisphenol A is another chemical that similar to methoxychlor, has the ability to bind estrogen receptors and initiate cellular responses similar to those caused by estrodiol. Recent findings include chromosomal damage in developing oocytes in mouse ovaries and abnormalities in the entire reproductive system in male mice, including a decrease in testicular sperm production and a decrease in fertility. In addition fetal exposure to bisphenol A increases the rate of postnatal growth and decreases the age at which females mature sexually. These females also have mammary gland abnormalities and appear pre-cancerous by the time the female reaches young adulthood. Bisphenol A also causes abnormal brain development, and change in brain function and behavior, similar to methoxychlor 46 . Ohi et al., 47 studied the adverse effects of Fipronil (phenylpyrazole) on reproductive function in Wistar rats and found that exposure lengthened the estrous cycle, decreased plasma progesterone and estradiol levels, and reduced the pregnancy index. Timing of exposure, duration, dose and susceptibility, or genotype of parent and fetus or child can play a role in the outcome observed. For each adverse reproductive effect, there appears to be a critical window of exposure. These concepts in reproductive and developmental human toxicity were discussed in detail in a series of workshops conducted by USEPA held in June 2000 4849 .
Neurodevelopmental abnormalities are exceedingly difficult to monitor, yet the evidence suggests that further investigation of time trends and causes is urgently needed. Chemical interference with hormone actions, particularly during neurodevelopment, and consequent effects on cognitive behavior are a current concern. Approximately 27% (696 of 2,588) of the workers who sprayed pure pyrethroids reported having experienced symptoms such as abnormal facial sensations (paresthesia), dizziness, headache, nausea, loss of appetite, blurred vision, and tightness of the chest. Eight of these workers were diagnosed with mild acute pyrethroid poisoning, characterized in part by listlessness and muscular fasciculations. GABA receptors are the target sites for several insecticides; including chlorinated cyclodienes (e.g. endosulfan) and phenylpyrazoles (e.g. fipronil). Parmigiani et al., 50 administered the widely used pesticide methoxychlor to pregnant mice and the offsprings were examined for neurochemical changes in the dopaminegeric system in the basal ganglia area of the brain. Neurons that use dopamine as a neurotransmitter (the dopaminegeric neural system) are involved in the control of locomotor activity and exploration. The basal ganglia were studied because one of the major impacts of methoxychlor on behavior is to increase exploratory activity to novel stimuli. This is also the area of the brain where degeneration occurs in Parkinson's disease, as well as associated changes in behavior. A change in behavior was associated, particularly in females, with a decrease in dopamine receptors in the basal ganglia. Males exposed to methoxychlor also showed an increase in territorial behavior, which is associated with very low levels of exposure to this pesticide. Low dose effects on the rat female reproductive system following exposure to a single administration of PBDE (Polybrominated diphenyl ethers) used as a flame retardants, observed a decrease in serum thyroxine (free) and serum estradiol concentrations in offsprings causes some neurobehavioural alterations such as nerve excitability and reproduction abnormalities 51 . Nerve excitability was assessed by presenting two sequential electrical stimuli of equal intensity and duration to the median nerve area of the wrist and recording the median nerve activity at the lateral side of the elbow. Lazarini et al., 52 also reported during the administration of deltamethrin 0.08 mg/kg of body weight, to a Wistar rat, persistent changes in behavior and/or biochemistry, including learning, motor activity, sexual behavior and they observed more learning behavior in males, zero effect on locomotion frequency in males or females. There is a relationship between exposure to certain neurotoxic pesticides such as pyrethroids during pregnancy and subsequent problems in children. Such a connection is suspected between learning and developmental disorders in children 53 . Certain pesticides may also interrupt the neurological development process particularly during critical period and induce harmful effects on sensory, motor and cognitive functions for example Aziz et al., 54 examined both the behavioral and biochemical changes by the administration of deltamethrin 1.0 mg/kg to the Druckrey rat. The effects were delayed surface righting reflex, during 6 and 12 weeks postnatal; increased acetylcholine esterase activity; increased GAP-43, regarding immunohistochemistry both % area and total number of positive cells were decreased. Experimental studies have shown that low-dose neonatal exposure to pesticides, including those in the organophosphorus category, may cause irreversible changes in the cerebral functions of adult animals 55 . Recent data concerning the effects of organophosphorus insecticides on the early development of the nervous system have prompted Health Canada and the United States Environmental Protection Agency (USEPA) to re-evaluate the toxicity of these products. It was on the basis of these new evaluations that severe usage restrictions were recently imposed for chlorpyrifos, an insecticide widely used in residential landscape maintenance.
In perhaps the most thought provoking recent work, Winrow et al. 56 demonstrated that inhibition of neuropathic target esterase (NTE) activity, either genetically or by OPs that inhibit NTE, results in a neurological hyperactivity phenotype in mammals (mice). Possible linkage of attention deficit/hyperactivity disorders in children to pesticide exposure in early childhood was hypothesized by these authors. This limited review of neurodevelopmental toxicants and their possible effects in children clearly indicate the need to conduct toxicogenomics studies together with neurobehavioral assessments of children and their exposures to pesticides.
Health Policy Activities
Recent activities (particularly by the USEPA and OECD) have been aimed at defining tiered testing strategies, validating screening and short-term tests and evaluating the feasibility of modifying existing test guidelines to encompass the detection of endocrine disrupting compounds. Finally, it is essential that a rigorous evaluation of assay is carried out prior to incorporation into a testing strategy and it is important to define how the information provided by these can be used in hazard identification and possibly for risk assessments 57 . At present, little effort is being made by public authorities to inform and sensitize the public about the rational use of pesticides. Accordingly, any major changes in the framework currently governing pesticide use should be accompanied by a comprehensive awareness campaign on health and environmental risks, so that the public fully understands the reason behind the changes. Furthermore, annual awareness campaigns should be developed by the relevant government departments. Regulatory agencies should recognize that the current endpoint of most tests to assess the risk of pesticides and other pollutants (carcinogenicity, acute toxicity, and immediate mutagenicity) have led to the misconception that these chemicals do not pose a threat to the health of wildlife, domestic animals, or humans. Low-level exposures to endocrine-disrupting compounds are ubiquitous in today's environment. Persistent chemicals such as DDT, PCBs and dioxins are detectable in nearly 100% of human blood samples, and even some of the shorter-lived potential endocrine disruptors are frequently detected in general population survey of residues in blood or urine 58 . The ubiquitous nature of the exposures combined with the nontrivial potential health effects justifies further research, education and preventive action to reduce human exposures to endocrine disruptors. A great deal of work on endocrine disruptors is taken by some of the international organizations are illustrated in Table 1.
The US Food Quality Protection Act (FQPA) of 1996 was dramatically altered the regulation of pesticides and other environmental pollutants in the United States. This Act, which amended both the Federal Insecticide, Fungicide and Rodenticide Act and the Federal Food Drug and Cosmetics Act, requires the USEPA to consider a number of factors relative to the toxicology of pesticides, including the cumulative effects of exposure to pesticides having a ‘common mechanism of toxicity'. The methods for identifying pesticides having a common mechanism of toxicity, and the ramifications of such a determination, are only now beginning to be clarified. Finally, implementation of the US FQPA (1996) by USEPA is expected to lead to reduction of the overall level of pesticide residuals in food. Included inthis effort is review of tolerances of pesticide levels in foods and efforts directed at increased protections for infants and children through additional acute, chronic, and neurotoxicity testing 59 . It is also essential to continue for examinig the transgenerational effects in animal studies because some pollutants require metabolism
Conclusion
The potential effects of endocrine-disrupting compounds (EDCs) on human health and the proven effects of EDCs on wildlife are a major concern among the public and the scientific communities. Ongoing efforts around the world to develop accurate biological monitoring of blood and urine for numerous chemical toxicants will improve exposure assessment in epidemiological studies and may eventually provide tools for physicians to assess risk to individuals 62 . As these tests become standardized and widely available, they will be useful clinically, just as blood lead testing has helped to facilitate a range of interventions that have resulted in a major reduction in lead poisoning.
Continue the Endocrine Disruptor Screening Program validation work, but combine this with a continuing assessment of the quality and efficiency of the screening assays being validated, as well as continuing improvement of the screening battery by adoption of better, more predictive assessment technology.Improve the Endocrine Disruptor Screening Program's prioritization process by developing a process that considers the potential to cause biological effects, rather than just exposure potential. Developing high-throughput pre screening (HTPS) assays to determine the cumulative risk assessment of compounds having endocrine disrupting activity. Developing new assessment and biomarkers to determine exposure and toxicity levels, especially how mixture of chemicals have impact on individuals or populations.
Different
Transgenerational exposure, hormonal activity, functionality, and delayed expression of effects must be addressed when determining the hazards of exposure to persistent chemicals already in the environment and of new compounds that might be released in the future. Mechanisms to inform the public about health risks and pesticide alternatives are also needed. Finally, knowledge development is another important goal, so that the risks and health effects of pesticides can be measured more accurately.
The public is routinely informed that the EDCs have been tested, that there are studies demonstrating the absence of their risk, and that regulatory agency adequately protect public health. Clearly significant changes are needed to bring current regulatory practices into conformity with new scientific information. We propose that testing for health effects at doses within the range of human exposure (currently not done) with respect to long-latency effects of developmental exposure throughout the lifespan (currently not done) be required prior to the introduction of any compound intended for use in commerce. Much of the research data reviewed here is necessarily descriptive. With the rise of genomics and toxicogenomics and the introduction of sensitive immunoassay and analytic methods capable of being used to measure toxicants levels in humans, mechanism-based human studies are at the horizon. With these tools, population toxicologists can assess differences and similarities between childrens and adults response to pesticide exposure.