Deforestation and Malaria in sub-Saharan Africa: an overview
C Uneke
Keywords
deforestation, malaria, mosquito, sub-saharan africa
Citation
C Uneke. Deforestation and Malaria in sub-Saharan Africa: an overview. The Internet Journal of Tropical Medicine. 2008 Volume 6 Number 1.
Abstract
Malaria remains the most complex and overwhelming health problem in sub-Saharan Africa. The disease is compounded by the generally poor social, environmental and economic conditions in the region. Most sub-Saharan African countries are experiencing unprecedented rate of population growth which has led to uncontrolled and unsustainable exploitation of natural resources, especially the forests resources. Through the process of forest clearing, deforestation alters every element of local ecosystems such as microclimate, soil, and aquatic conditions, and most significantly, the ecology of local flora and fauna, including malaria vectors. Mosquitoes are highly sensitive to environmental changes because of deforestation: their survival, density, and distribution are dramatically influenced by small changes in environmental conditions, such as temperature, humidity, and the availability of suitable breeding sites. Changes in mosquito ecology and human behaviour patterns in deforested regions influence the transmission of malaria and deforestation has therefore been shown to increase the risk of malaria transmission in sub-Saharan. Because deforestation is a process that cannot be readily controlled for a variety of political and economic reasons, investigations and assessments of possible impacts of future deforestation will be crucial to minimize the ecological degradation caused by human activities and to control epidemics of malaria.
Introduction
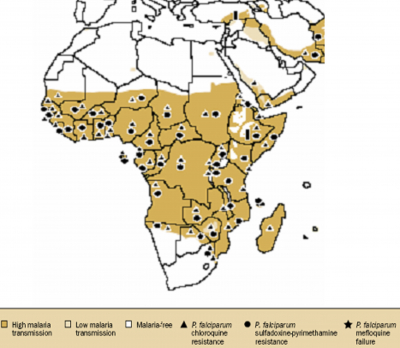
Malaria remains the most complex and overwhelming health problem, facing humanity with 300 to 500 million cases and 2 to 3 million deaths per year 1 . About 90% of all malaria deaths in the world today occur in sub-Saharan Africa (Figure 1) and this is because majority of infections are caused by
Most sub-Saharan African countries are experiencing unprecedented rate of population growth without any appreciable improvement in the socio-economic status of the populace. There is therefore a high level of poverty and underdevelopment in the region particularly in the rural areas. One major consequence of this situation is uncontrolled and unsustainable exploitation of natural resources, especially the forests resources, which has generated severe environmental, ecological and public health problems including increase in vector-borne diseases like malaria. Forests are among the world’s most important biomes in terms of the area of land surface they cover (approximately 30% of all land - over 3.8 billion hectares 8 , and the goods and services they provide, and the biodiversity they contain (approximately 90% of terrestrial biodiversity) 9 . More than 1 billion people depend on forests for their livelihoods to varying degrees and 60 million indigenous people are almost wholly dependent on forests, while around 350 million people living within or adjacent to dense forests depend on them to a high degree for subsistence and income, in developing countries, agro-forestry farming schemes support 1.2 billion people and help sustain agricultural productivity and the generation of income 10 . Forest industries provide employment for some 60 million people worldwide 9 .
Through the process of clearing, deforestation alters every element of local ecosystems such as microclimate, soil, and aquatic conditions, and most significantly, the ecology of local flora and fauna, including human disease vectors 11 . Of all the forest vector species that transmit diseases to humans, mosquitoes are among the most sensitive to environmental changes because of deforestation: their survival, density, and distribution are dramatically influenced by small changes in environmental conditions, such as temperature, humidity, and the availability of suitable breeding sites 121314 . Changes in mosquito ecology and human behaviour patterns in deforested regions influence the transmission of mosquito-borne diseases such as malaria, Japanese encephalitis, and filariasis 1516 . In particular, each incident of deforestation and land transformation has a different influence on the prevalence, incidence, and distribution of malaria directly and indirectly 15 . Numerous country and area studies have described the influence of deforestation and subsequent land use on the density of local mosquito vectors. Some of the studies were able to follow-up further and observe changes in local malaria incidence and prevalence due to the mosquito density change 111516 . Thus malaria transmission (and also the control) has clear links to ecosystem changes that result from natural resource policies such as land tenure, road building, and agricultural subsidies; and deforestation is a major development policy concern which often heralds many other ‘malaria-causing’ land use changes 17 .
Despite the high rates of malaria and deforestation in sub-Saharan Africa, the relationships between both contextual challenges have not been comprehensively assessed. The objective of this report therefore is to evaluate the relationship between deforestation and malaria in sub-Saharan Africa with the view to providing information that has policy relevance as it concerns malaria control and sustainable forest resource exploitation in sub-Saharan Africa. To achieve this, a Medline Entrez-Pubmed search was performed and studies conducted in sub-Saharan Africa and reported in English which investigated deforestation and malaria were identified. Combinations of key words such as
The burden of malaria in sub-Saharan Africa
Malaria threatens the lives of 40% of the world’s population – over 2.2 billion people 18 . The World Health Organization and the World Bank rank malaria as the largest single component of the disease burden in Africa, causing an annual loss of 35 million future life-years from disability and premature mortality. In Africa, malaria is responsible for about 20-30% of hospital admissions and about 30-50% of outpatient consultations 18 . Sub-Saharan Africa still remains the most malaria endemic area in the world (Figure 1). The global impact of malaria is staggering, regardless of how it is measured. Biomedically, the burden of disease is a function of many things and various public health disciplines define it differently. Epidemiologists refer to burden in terms of morbidity and mortality, while economists refer to a quantification of the costs (direct and indirect), and effects on levels of productivity, national growth, and development 19 . The cost of malaria can be measured in lives lost, in time spent ill with fever, and in economic terms. Money spent on preventing and treating malaria, the indirect costs of lost wages, time home from school, and time spent caring for sick children, adds up at the personal level. In most countries in sub-Saharan Africa, in the public sector, large fractions of health sector budgets are spent on malaria control and treatment and at the macroeconomic level, a heavy national burden of malaria dampens economic development, sometimes subtly, but pervasively. All of these effects are recognized and accepted widely, but their magnitude has been poorly documented.
Figure 1
In areas where malaria is highly endemic, a protective semi-immunity against
In sub-Saharan Africa, malaria is currently known to enhance the susceptibility of infected individuals to other infectious agents. One outstanding instance is the co-infection of malaria with HIV infection. Concerning the global HIV epidemic, sub-Saharan Africa remains by far the worst-affected region with 25.4 million people living with HIV (Just under two thirds, i.e. 64% of all people living with HIV) 26 . Because of the high prevalence of malaria and HIV in sub-Saharan Africa, co-infections are common. Initial studies conducted among children and adults failed to show a consistent pattern of a biologic or clinical interaction between malaria and HIV infection 272829 , the two diseases were however, identified to critically intersect in pregnancy and have serious consequences in pregnant women, their fetuses, and infants 3031 . Approximately one million pregnancies per year are thought to be complicated by coinfection with malaria and HIV in sub-Saharan Africa 30 .
Beyond mortality, malaria causes morbidity through fever, weakness, malnutrition, anemia, spleen diseases and vulnerability to other diseases. The health consequences of malaria vary in terms of severity, but the global impact of malaria on human health, productivity, and general well-being is profound particularly in sub-Saharan Africa 38 . The joint mortality and morbidity impacts of malaria are estimated to be 45 million DALYs (disability adjusted life years) in 2000 or nearly 11% of all infectious diseases 39 . Malaria imposes substantive social and economic costs and impedes economic development through several channels, including but certainly not limited to, quality of life, fertility, population growth, saving and investment, worker productivity, premature mortality and medical costs in sub-Saharan Africa 3840 . Malaria is estimated to cause a decline in economic growth in the range of 0.25% to 1.3% of per capita GNP growth in tropical countries, even after accounting for initial endowments, overall life expectancy and geographic location 3940 . To the extent that slow economic growth limits funds for malaria control, there is a vicious cycle of poverty and malaria that diminishes economic opportunities for a large number of the world’s inhabitants especially in sub-Saharan Africa 17 .
Forest resources and their exploitation in sub-Saharan Africa
The malarious regions of the world are among the areas with the largest global forests. Although estimates of the global extent of humid tropical forest vary greatly, from 11.16 million to 15.71 million km 2 , the largest surviving areas of such forest are to be found in Latin America (6.53 million– 7.80 million km 2 ), chiefly in the Amazon region, followed by Africa (1.93 million– 5.19 million km 2 ) and South–east Asia (2.70 million–2.72 million km 2 ) 414243 . Both forest biodiversity, and the natural functioning of health forest ecosystems, contribute immensely to human health. Forest species contribute to balanced diets, and are a major component of traditional healthcare systems – which provide the primary healthcare for around two-thirds of the global population 44 . The medical needs of approximately one billion people depend on drugs derived from forest plants, many of which have been long been used in traditional medicine. In recognition of the importance of forest ecosystems and the services provided, the proportion of forest cover to total land area (excluding inland waters) has been adopted as an indicator measure for monitoring progress towards achieving the Millennium Development Goals (MDGs) adopted by the member states of the United Nations under the 2000/2002 Millennium Declaration 45 . It is already well established that the drastic modification of the environment as a result of overexploitation of natural resources particularly the forest resources, results to severe health, ecological and economic consequences especially among the rural populace in sub-Saharan Africa and other tropical regions 46474849 .
Deforestation in malarious areas is extensive, with global estimates of its rate ranging from 36,000–69,000 km2/year 50 . The estimated rates of global deforestation are astonishing. According to the World Resources Institute (http://pubs.wri.org/pubs_content.cfm?PubID=3018), more than 80 percent of the Earth's natural forests have already been destroyed (at a rate of about 40 million hectares per year). Up to 90 percent of West Africa's coastal rain forests have disappeared since 1900. Loss of habitats is among the obvious consequences of deforestation` (seventy percent of the Earth's biodiversity is present in forests). Rain forests help generate rainfall in drought-prone countries elsewhere. Studies have shown that destruction of rain forests in such West African countries as Nigeria, Ghana, and Côte d'Ivoire may have caused two decades of droughts in the interior of Africa. (http://pubs.wri.org/pubs_content.cfm?PubID=3018). Analysis of the Food and Agriculture Organization of the United Nations (FAO) report shows that developing countries in the tropics generally suffered the worst rates of forest loss between 2000 and 2005. Of the 10 countries with the highest deforestation rate during that period, all were considered “developing” and nine were tropical. Four of the top six were located in south or southeast Asia as follows; Nigeria 55.7%, Viet Nam 54.5%, Cambodia 29.4%, Sri Lanka 15.2%, Malawi 14.9%, Indonesia 12.9%, North Korea 9.3%, Nepal 9.1%, Panama 6.7%, Guatemala 6.4% 51 . Nigeria therefore, has the world's highest deforestation rate of primary forests according to the revised deforestation figures from the FAO and between 2000 and 2005 the country lost 55.7 percent of its primary forests -- defined as forests with no visible signs of past or present human activities 51 .
Most of Africa’s deforestation is caused by slash-and-burn agriculture. Forests are rarely replanted as people move from depleted lands to clear new forest areas. Yet forests and trees make an essential contribution to rural life by providing firewood, dyes, fruits, nuts, and building materials. Firewood and charcoal represent more than half the energy consumption in sub-Saharan Africa 52 . The fight against deforestation is being lost in some countries. For example, Côte d’Ivoire once had the largest rainforest in West Africa. In 2000, deforestation reduced the coverage of forests to 22 percent of the country’s area 53 . Hope comes from such countries as Gambia and Rwanda, which have been praised for their efforts to save and replant forests. In 2004, the World Bank approved a $40 million grant to help Madagascar preserve natural forests and protect biodiversity 54 .
Rapid growth in population and expansion in socio-economic development in sub-Saharan Africa, are imposing considerable pressures on the natural resources base and the environment in the region. There is a need to satisfy rapidly growing demands for agriculture, housing, and infrastructure development. This has led to environmental degradation manifested as deforestation, and loss of wildlife habitat, desertification, erosion, floods and general loss of the productive land base of the countries of sub-Saharan Africa. Deforestation is driven by a wide variety of human activities, including agricultural development, logging, transmigration programs, road construction, mining, and hydropower development 10 . Meanwhile health, poverty and forest degradation remain very closely intertwined, with many of the world’s poorest people depending directly on a wide range of forest resources and services for their livelihoods 4649 . The effects of deforestation on ecosystems and human health are diverse and have taken place for many decades, though both the rate and geographic range have increased markedly over the last 30 years 15 . Severe forest disruption via over exploitation (deforestation), combined with evolution (of pathogens), globalization (in trade and travel) and changes in living patterns, can therefore lead – and have already led – to the loss of many forest goods and services, with profound implications for human health particularly the transmission of vector borne-diseases like malaria 1547 .
Deforestation and malaria: the interconnecting link
Malaria is a major killer and factor in the burden of disease in and near forested areas in Africa. An interesting hypothesis, hotly debated in the current literature 555657 is that when Africans experienced a dramatic change in their environmental and behavioural practices 10,000 years ago, shifting from hunter-gatherers to farmers, malaria started to infect humans 58 . Forest clearing either for farming or other purposes has allowed populations to enter areas that malaria had previously rendered uninhabitable. The causal links between deforestation and incidence of malaria are difficult to distinguish. Some logging processes can lead to standing water and increases in mosquito breeding sites. Road building, tree felling, reduced shade and increased pooling of water have been shown to promote breeding and more rapid development of mosquito larvae 5960 . Of additional concern, a form of malaria previously found in non-human primates has recently been found in humans in Southeast Asia 6162 .
Massive clearing of forests has enormous impacts on local ecosystems and human disease pattern. It alters microclimates by reducing shade, altering rainfall patterns, augmenting air movement, and changing the humidity regime 63 . It also reduces biodiversity and increases surface water availability through the loss of topsoil and vegetation root systems that absorb rain water 64 . For anopheline species that breed in shaded water bodies, deforestation can reduce their breeding habitats, thus affecting their propagation. On the other hand, some environmental and climatic changes due to deforestation can facilitate the survival of other anopheline species, resulting in prolonged seasonal malaria transmission 16 . Deforestation and land transformation influence the malaria vector anophelines, especially larval survivorship, adult survivorship, reproduction and vectorial capacity, through changing environmental and microclimatic conditions such as temperature (average, variability), sunlight (amount, duration), humidity, water condition (distribution, temperature, quality, turbidity, current), soil condition, and vegetation 65 .
In the western Kenya highlands it was reported that Mosquitoes that were placed in houses in the deforested area showed a 64.8–79.5% higher fecundity than those in houses located in the forested area, but the median survival time was reduced by 5–7 days 66 . According to the report female mosquitoes in the deforested area showed a 38.5–40.6% increase in net reproductive rate and an 11.6–42.9% increase in intrinsic growth rate than those in the forested area; significant increases in net reproductive rate and intrinsic growth rate for mosquitoes in the deforested area suggest that deforestation enhances mosquito reproductive fitness, increasing mosquito population growth potential in the western Kenya highlands. The vectorial capacity of
Many species of mosquitoes prefer to feed on animals rather than humans. Destruction of animal habitats, and a decline in the number of wild animals forces the mosquitoes to feed on domestic animals and humans 15 . The frequency of human feeding has a marked influence on malarial transmission. Consequently, in most places the clearing of land results in an increase in malaria. Anopheles mosquitoes that breed in partially shaded or sunlit water replace the forest-breeding mosquitoes. In some cases these mosquitoes are more efficient vectors than those replaced. Deforestation practice therefore can result in a massive increase in mosquito breeding sites. Dragging logs through the forest can cause water-filled furrows. Tire marks, hoof prints, and even human footprints, can provide ideal breeding places. Removal of growth along stream edges, slowing of water run off by debris, and impoundment for water supplies, accumulation of coconut shells, tins, tires, and other rubbish, and pooling of water in tree stumps, all increase the breeding potential 15 .
The relationship between malaria transmission, forest cover and deforestation is complex. Aspects related to microclimate and/or the chemical composition of soils can be important 69 . Ecological factors can regulate the species composition of the mosquito populations, and thus the numbers and types of malaria vector, by, for example, changes in host-preference and predation patterns 70 . Human population migrations to and from forests (usually driven by economic and social pressures) and the associated changes in land cover are often critical 15 . Such migrations often bring human populations closer to the forest. The direction of land-use that follows forest clearing— usually towards grasslands or crops — is also important but its influence will be mediated by the local ecology and vectors 16 . The replacement of forest with rice cultivation, for example, may provide more favourable conditions for
A synthesis of the literature on forests and malaria proposes at least six pathways through which deforestation can affect malaria infection and disease 76 .
(i).
(ii).
(iii).
(iv).
(v).
(vi).
In the process of deforestation and subsequent land transformations, improved surveillance and monitoring are necessary to detect changes in the environment, vector density, human migration and behaviour, and malaria incidence to prevent further deterioration of malaria status in the region.
Public Health Consideration
Malaria is a major threat to public health and economic development in Africa. It is a disease of poverty and under-development and in sub-Saharan Africa malaria disproportionately affects the poorest of the poor populations including the forest communities which are often excluded from development processes. Reaching the poorest of the poor with malaria control interventions poses great challenges, not solely because of financial barriers to accessing care and prevention services but also because the poorest populations often live in the most remote areas and are socially or culturally marginalized 82 . Because forests, almost by definition, have lower population densities than urban areas, people living in forest and rural areas under threat of deforestation tend to be disregarded in formal health care systems including systematic malaria interventions. In most parts of the sub-Saharan Africa, these areas are often difficult to reach, and remote forested areas may have difficulty attracting doctors, nurses and health system administrators. There are ethical and practical reasons for reversing this trend.
In the global development community, concerns that public health interventions may not be reaching poor and marginalized populations have led investigators to examine the differences in the burden of disease and the coverage and impact of public health interventions among persons with differing socioeconomic status 83 . If extra efforts are not made to reach the poorest of the poor with effective malaria control interventions, it is very likely that the Roll Back Malaria (RBM) target of reducing the global malaria burden by 50% by the year 2010 will not be reached particularly in sub-Saharan Africa. Interventions, therefore, must be designed to ensure that a large percentage of the most poor are using effective treatment, insecticide-treated bed nets (ITNs), and other essential malaria control interventions 82 .
Because deforestation is a process that cannot be readily controlled for a variety of political and economic reasons, investigations and assessments of possible impacts of future deforestation will be crucial to minimize the ecological degradation caused by human activities and to prevent epidemics of malaria and other vector-borne diseases 65 . Another challenge of deforestation to malaria control is loss of plants that could provide new treatments for malaria and other diseases. It was demonstrated in Ekiti State, Nigeria how botanicals used by local populations for treating malaria are becoming rarer 84 . The problem arises because of a land tenure system that pushes the boundaries of farms into the forests. In looking at the economic burden of malaria we talked about the need to integrate malaria control and development planning. Now we can see that environmental management must also be a strong part of that picture 78 .
An additional challenge with malaria is that it has developed resistance to some antimalarial drugs. Also, newer and more effective antimalarial drugs are expensive. A 2003 survey in 28 African countries showed that only 42 percent of children with malaria were treated with antimalarial drugs. Most were treated with less effective drugs because they were less expensive. In Tanzania, the cost of malaria treatment was the largest household expenditure. Only in five countries, Botswana, Djibouti, Namibia, South Africa, and Swaziland, is malaria treatment free 85 . In 1997, the WHO, the World Bank, and other international agencies launched the Multilateral Initiative on Malaria. This program seeks to promote research and develop strategies to control the disease. Its main focus has been on Africa. In 1999, the trial use of insecticide-treated bed netting (ITNs) in Burkina Faso, Gambia, Ghana, and Kenya reduced malaria deaths of children there by 25 to 40 percent 86 . Since then, other African countries have made an effort to provide people with ITNs. However, at the end of 2004, fewer than 5 percent of African children were sleeping under an insecticide-treated net. Many poor households cannot afford the net, which costs between $2 and $5 87 .
Since societal response to malaria is important and essentially consists of preventive (control) and curative (treatment) approaches; a recent study noted that the efficacy of both is affected by the mediating role of ecological and behavioural factors at the individual, community, and regional levels 17 . The main form of prevention is vector control, that is, destroying mosquito habitat and mosquitoes themselves at various stages of their life cycle by clearing vegetation, modifying river boundaries, draining swamps, applying oil to open water bodies, screening houses, and spaying insecticides (e.g. DDT). Individuals can also engage in preventive behaviours by using insecticide treated bednets and minimizing exposure by controlling activities in the early morning and evening hours 17 . The report of a WHO study group on malaria vector control and personal protection in 2006 88 , emphasized the need for the following: (i). To sensitize the general public and the local authorities about the ravages of malaria on the health of native forest populations and the need to collaborate in the preservation of their environment, while ensuring accessibility to health care and education facilities; (ii). To strengthen and diffuse mechanisms of public information about the existence of health care posts open to everybody, in order to reach population groups who may be reluctant to use public health services; (iii). To ensure that malaria workers and other health staff recognize the importance of information about new economic activities in their areas of responsibility, as well as population movements and occurrences of fever outbreaks, which should require immediate reporting and investigation; (iv). To promote and support epidemiological and entomological research into the ways of penetration, attraction factors and mechanisms of adaptation of forest vectors to human hosts and shelters, as well as the colonization of forest fringe tree plantations by forest vectors, such as
Some researchers have made a compelling argument for an integrated approach to malaria control that relies on environmental management at its core 89 . The researchers argue that although society is engaged in a wide variety of efforts to combat malaria, including new drugs, engineered malaria-resistant mosquitoes and vaccines, these efforts will take time and are not guaranteed to deliver especially in sub-Saharan Africa with its current high level of poverty. In the interim, the best option appears to lie in environmental management for vector control, including vegetation clearance, management of water bodies (e.g., modification of river boundaries, drainage of swamps, reduction of standing water, application of oil to open water bodies), and use of screens and bed nets 17 .
Since the resources required for the accelerated expansion of health facilities and services to the rural areas in sub-Saharan Africa where the malaria burden is greatest are beyond the financial capacities of most countries, it is necessary that many interventions be undertaken by the communities themselves. This is already happening. A large number (12-82%) of all malaria episodes in sub-Saharan Africa are now managed outside the official health sector 9091 . The private sector now accounts for 40-60% of all antimalarial drugs distributed, with unofficial sources, such as street sellers and market stalls, accounting for as much as 25% 92 . One of the principles of the RBM movement is that it is driven by community priorities, in particular the protection and care of women and children. Studies have shown that ITN use has a protective efficacy of 17%, saves about six lives each year for every 1000 children protected, and reduces the incidence of mild malaria episodes by 48% 93 . Community-based malaria projects have been shown to be both efficacious and feasible. A number of small-scale projects have demonstrated that community-based interventions based on training of caregivers (e.g. mothers) to provide early diagnosis and treatment has great potential 94 .
It is pertinent to state that deforestation is an integral part of the life of forest communities in rural sub-Saharan African countries, therefore any malaria control effort that ignores the role of deforestation and its associated ecological and behavioural dimensions may suffer from serious setbacks. Conservation policies aimed at slowing deforestation will impact malaria as observed in a number of previous studies 154995 . Sustainable forest management is an important element of local development policies, as donor agencies and local policy makers seek to take a more integrative view of people in the natural landscape and the resulting changes in land cover, as well as changes in how people interact with the forest, these have implications for malaria 17 .
Just as effective community participation is imperative for the success of malaria intervention, so it is in the evolution and implementation of forest conservation and management policies. The role of forestry communities in forest management is presently almost non-existent in most parts of Africa. In some states of southern Nigeria particularly Cross River and Edo States, communities are now involved in forest management following the evolution of new forestry sector strategies. In these States, Forest Management Committees from the local communities are involved in the management of plantations and natural forests in both reserve and off-reserve areas. The communities living in the immediate vicinity of the forest resources in these States have a higher motivation to assure a sustainable management if their future rights to the resources are legally secured. Likewise, these local communities can assure a cheaper and more effective control and monitoring of a sustainable management system than can be achieved by the government 96 . If implemented in sub-Saharan Africa, the strategy has the long term objective of assuring the conservation and rational utilization of the forest resources through improved protection and management and the sustainable exploitation of both timber and non-timber products. At the same time, the socio-economic benefits that the local people would derive from the forest will be increased and secured. This would minimize unsustainable and uncontrolled forest exploitation which would go a long way to reducing the increasing incidence of deforestation-dependent malaria epidemics.