Pictorial Essay Of High Resolution And Colour Doppler Sonography Of Scrotal Pathologies.
B M V, P Setty
Keywords
colour doppler, high-resolution sonography, scrotal pathology
Citation
B M V, P Setty. Pictorial Essay Of High Resolution And Colour Doppler Sonography Of Scrotal Pathologies.. The Internet Journal of Radiology. 2009 Volume 12 Number 2.
Abstract
High-resolution sonography is a simple, widely available, inexpensive and non-irradiating, noninvasive, practicable, repeatable, enables rapid evaluation and is widely accepted as the method of choice for screening and diagnosis of spectrum of scrotal pathologies. When color Doppler and power Doppler imaging are added, testicular perfusion can be assessed, [1] which provides valuable information in assessment of the acute painful scrotum in addition to scrotal masses and male infertility. This pictorial essay reviews a spectrum of appearance of common and rare cases scrotal pathologies.
Introduction
Because of superficial location of scrotum and rapid technological advancements and High-resolution sonography color Doppler provides a very good details of anatomy of the scrotal wall, testis, epididymis and testicular perfusion. It is widely accepted as the method of choice for screening and diagnosis of scrotal diseases. In this pictorial review, sonographic findings of a wide variety of scrotal lesions are presented; imaging findings of intratesticular tumors, benign intratesticular lesions, extra testicular tumors, inflammatory and ischemic lesions, and conditions such as hematoma, inguinal hernia and undescended testis are presented.
Materials and Methods
All cases were performed using standard USG machine (Philips Envisor CHD, Netherlands USA) equipped with high resolution and colour Doppler linear probe of 7.5-12 MHz. Examination was performed in supine position with a folded towel positioned between the patient’s legs to support the scrotum. Serial transverse and sagittal images of each testis and epididymis are obtained and both testicles are compared in echo texture and colour flow.
Normal US Scrotal Anatomy
A normal adult testis is oval shaped, measures 5 × 3 × 2 cm in size and has homogeneous and intermediate echogenicity [Fig.1A].
Figure 1
The tunica albuginea, a dense fibrous capsule deep to the tunica vaginalis, it is reflected into the interior of the testis, forming the incomplete septum along the longitudinal axis of the testis known as the mediastinum of the testis [Fig1B].
Figure 2
The epididymis, which overlies the superolateral aspect of the testis, comprises a head, body, and tail. The tail of the epididymis continues as the vas deferens in the spermatic cord. The epididymal head measures 5–12mm in size, body of the epididymis is 2–4 mm thick. Testicular appendages such as the appendix testis [Fig 1C], a müllerian duct remnant found at the superior aspect of the testis, and the appendix epididymis, is a mesonephric remnant located at the epididymal head [Fig 1D]. [2]
Figure 3
Figure 4
The spectral waveform of the intratesticular arteries has a low-flow, low-resistance pattern [Fig.1E] with a mean resistive index of 0.62 and peak systolic velocity ranges from 4 to 19cm/s.
Scrotal Wall Cellulitis
Scrotal wall cellulitis is common in patients who are obese, diabetic, or immunocompromised. The ultrasound (US) signs are an increase in scrotal wall thickness and the presence of hypoechoic areas with increased blood flow seen at color Doppler. [Fig .2]
2.Scrotal wall cellulitis. Transverse view of simultaneous both gray scale and power doppler of scrotum shows of scrotal wall oedema (E) ,thickened median raphae (M), with reactive hydrocele (H) on both sides, power Doppler image shows increased flow (arrow).
Inguinal and Scrotal Swelling
Inguinal Hernia
US is helpful in patients with equivocal physical findings and in those presenting with acute inguinoscrotal swelling. Hernias are classified as direct or indirect, depending on their relationship to the inferior epigastric artery by using color Doppler US [5]. In hernial sac contains most commonly bowel loops, [Fig.3A] next most common content is omentum, which appears as hyperechoic areas in US [Fig 3B]. In real time US an akinetic dilated loop of bowel in the hernial sac is, hyperemia of bowel wall and scrotal skin are suggestive of strangulation [5].
Figure 6
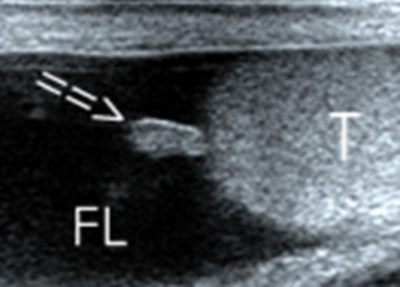
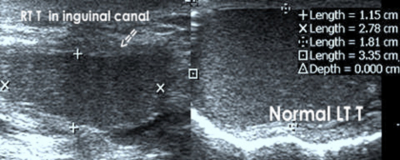
Cryptorchidism
The testes develops in the retro peritoneum and descend downward through the internal inguinal ring, inguinal canal, and external inguinal ring to the scrotum. Malpositioned testes may be located anywhere along the pathway of descent from the retroperitoneum to the scrotum, but the majority of undescended testes (80%) are palpable and will be found at high inguino scrotal region, amenable for localization of a testis easily and rapidly by Ultrasound.[6] Undescended testis is most commonly seen in male infants, bilateral in10% to 33% [7] [Fig 4A,4B,4C].The cryptorchid testis is usually smaller and isoechoic or hypoechoic relative to the normally located testis. [Fig 4D]
Figure 10
Figure 11
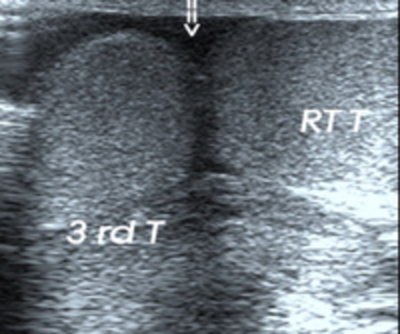
5A.Polyorchidism.Longitudinal grayscale US shows, third testis (asterix) located on right side superior and lateral to right testis (RT),with absent median raphae (arrow), associated with indirect inguinal hernia with herniated bowel loop (arrow head).
Polyorchidism
It is defined as presence of more than two testes, is a rare developmental anomaly of the genital tract, with approximately 70 cases reported [8]. The most popular, theory of its origin is due to duplication or abnormal division of the urogenital ridge [9]. A majority of supernumerary testis located on left side and lacks its own epididymus in 90 %. Its associations with cryptorchidism, indirect inguinal hernias, testicular torsion, hydrocoele, epididymitis, varicocoele and infertility have been reported [10] [Fig 5A, 5B].
Figure 12
Figure 13
Hydrocele
Hydrocele is an abnormal collection of serous fluid accumulating between the visceral and parietal layers of the tunica vaginalis .On US appears as an anechoic fluid collection surrounding the anterolateral aspects of the testis. A hydrocele may be Primary due to idiopathic cause [Fig 6A]; Secondary hydrocele occurs following scrotal trauma or secondary to epididymitis, torsion, or neoplasm [Fig 6B].
Figure 14
Figure 15
Congenital hydroceles result from a patent processus vaginalis that permits entry of peritoneal fluid into the scrotal sac is the most common cause of painless scrotal swelling in children [Fig 6C].
Pyocele
A pyocele results from untreated epididymo-orchitis or rupture of an intratesticular abscess into the space between the layers of the tunica vaginalis. In US it appears as complex cystic lesions with internal septations and loculations. Skin thickening and calcifications can be seen in chronic cases. [Fig.6D]
Conditions of the Spermatic Cord
Spermatic cord hydrocele
Spermatic cord hydrocele (SCH) is a rare congenital anomaly, resulting from an abnormal closure of the processus vaginalis. It is a loculated fluid collection along the spermatic cord, located above the testicle and the epididymis. Two types of SCH are recognized [12]. The first type is encysted hydrocele of the cord, where the fluid collection does not communicate with the peritoneum or the tunica vaginalis [Fig 7A, 7B]. The second type is the funicular hydrocele, where there is a fluid collection along the cord, communicating with the peritoneum at the internal ring [Fig 7C].
Figure 18
Figure 19
Varicocele
A varicocele is an abnormal dilatation of the pampiniform plexus of veins secondary to the incompetent valves in the internal spermatic veins. The color Doppler US is nearly 100% sensitive and specific in varicocele detection. [3] Primary or idiopathic is the most common type of varicocele, left side more common, present in approximately 15% of adult men between the ages of 15 and 25 years. Criteria for diagnosis of varicocele are (a)In gray-scale US the largest vein measured more than 2 mm in diameter in supine position or more than 3 mm in diameter in standing; (b) increase in more than 1 mm size during valsalva [Fig 8A]; (c) In color Doppler US reflux more than 2-sec during valsalva maneuver [Fig 8B] [14]. A combination of the (a) & (b) or (a) &(c) criteria are used. Grading of Varicocele based on Doppler reflux during valsalva : grade 1, static reflux (<2 s); grade 2, intermittent reflux (>2 s); and grade 3, continuous reflux or reflux during normal respiration.[14]Secondary varicoceles are less common and occurs in the elderly, secondary to retroperitoneal disease processes, renal cell carcinoma with left renal vein thrombosis.
Figure 21
Tumors of the Spermatic Cord and paratesticular tissues
Lipoma is the most common benign tumors of the spermatic cord. Sarcomas are most common malignant neoplasms of the paratesticular tissues. Paratesticular rhabdomyosarcoma is one of the most common non-germinal neoplasms affecting the scrotal contents in children and young adults. In US, mixed echogenicity extratesticular mass with hypervascularity, epididymus may be obscured by the mass. [17] [Fig 9]
Figure 23
Epididymo-orchitis (EO).
EO is a common cause of acute scrotum. The epididymal head is the most commonly affected region. In acute EO affected organ shows increased size, decreased echogenicity and reactive hydrocele and wall thickening are frequently present [Fig 10A]. In colour Doppler US the hallmark of scrotal infection is hyperemia of the epididymis, testis, or both is a well-established criterion for the diagnosis of EO [18] [Fig 10B]. In acute EO, pulse wave Doppler shows high-flow, low-resistance wave pattern with the resistive index is less than 0.5 [19, 20, 21] [Fig 10B]. Considering peak systolic velocity more than 15 cm/sec, the diagnostic accuracy for orchitis is 90% and 93% for epididymitis [22] [Fig 10C]. Complications of acute EO include chronic pain, infarction, [Fig 10D] abscess, pyocele, gangrene, infertility, and atrophy.
Figure 25
Figure 26
Figure 27
Figure 28
Chronic EO most commonly results from untreated or incompletely treated acute EO can occur. The affected part of the epididymis, and testis, is enlarged with heterogeneous echogenicity, prominent septations and calcifications [23] [Fig.11A]. At color Doppler imaging, chronic EO may not demonstrate the increased blood flow typical of acute epididymitis.
Tuberculous epididymo-orchitis
The tuberculous bacillus can also gain entry via the hematogeneous and lymphatic routes. With both direct and hematogenous spread, the tail of the epididymis is usually the first structure to be involved, due to its high vascularity.[25,26,27] On US the following types: diffusely enlarged, homogeneously hypoechoic; nodular enlarged, heterogeneously hypoechoic; or military. Evidence of tuberculosis infection elsewhere, failure of conventional antibiotic therapy, and scrotal calcifications, abscess, and sinus tract are helpful clues in aiding the diagnosis of tuberculosis epididymitis and tuberculosis epididymoorchitis [24] [Fig.11B, 11C].
Figure 30
{image:31}
Testicular Torsion
In testicular torsion, due to twisting of the spermatic cord, early diagnosis is most critical because, testicular salvage is more likely if surgery done within 4–6 hours after the onset of torsion. Two types of testicular torsion (a) Extravaginal torsion most common in newborns and (b) Intravaginal torsion is more common in adolescents in with a predisposing factor of the “bell clapper” deformity, in which tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate. In the acute torsion, testicular echogenicity may appears normal later on diffuse decrease in echopattern with enlargement [Fig.12 A].
{image:32}
In sub acute torsion (1 to 10 days), enlarged testis and heterogeneous echogenicity, absent flow in testis with increased surrounding vascularity [Fig 12B]. Sonographic evaluation of the spermatic cord is important as the point of cord twisting can be identified at the external inguinal orifice called “whirl pool sign” is the most definitive sign of torsion because it has 100% specificity and sensitivity [28][Fig12C]. The intrascrotal portion of the cord appears as edematous, round, ovoid or curled echogenic extra testicular mass, with the epididymal head wrapped around it [Fig 12D,12E].The definitive diagnosis of complete testicular torsion is made when blood flow is visualized on the normal side but is absent on the affected side [Fig 12F].
{image:33}
{image:34}
{image:35}
{image:36}
{image:37}
Incomplete torsion refers to cord twisting of less than 360°, in which some arterial flow persists in the affected testis; it is important to compare the two testes by using transverse views, in which colour Doppler shows reduced flow, with additional pulsed-wave Doppler imaging, decreased or reversed diastolic flow may be evident on the affected side [Fig 12 G, 12H, 12I].
{image:38}
{image:39}
{image:40}
Scrotal Tumors
Patients usually presents as painless scrotal swelling. Because of excellent spatial resolution US can differentiation between intra or extra testicular and solid from cystic tumors , it is nearly 100% sensitive for identifying scrotal masses [29].
Extratesticular Tumors
Adenomatoid tumor is the most frequent extra testicular tumor. It is benign, occurs in the age group of 20 to 50 yrs, frequently arises from the poles of the epididymis most common at tail [1] [3]. They are generally unilateral, smooth, round, well circumscribed echogenic mass with minimal vascularity and rarely measuring more than 5 cm [Fig13A, 13B, 13C].
{image:41}
{image:42}
{image:43}
{image:44}
Epididymal cysts
They are not true tumors but usually manifest as a palpable mass. They contain clear serous fluid; they are seen as an anechoic, well-defined cystic lesion with increased through transmission [Fig14A, 14B].
{image:45}
{image:46}
Spermatocele
They represent cystic dilatation of tubules of the efferent ductules in the head of the epididymis. Spermatoceles are usually unilocular but can be multilocular. At US examination, they are well-defined hypoechoic lesions usually measuring 1–2 cm and demonstrating posterior acoustic enhancement, with low-level echogenic proteinaceous fluid and spermatozoa [Fig15 C].
{image:47}
Malignant Testicular Tumors
Seminoma
Approximately 95% of malignant testicular tumors are germ cell tumors, of which seminoma is the most common histological subtype. Compared to the nonseminomatous germ cell tumors, seminoma occurs in an older patient population, with a mean age of approximately 40 years. On gray-scale US scans, appears as a homogeneous hypoechoic lesion, which corresponds to uniform appearance of the gross specimen [Fig 16 A]. On colour Doppler larger lesions shows increased vascularity. There can be multifocal or unifocal lesions [Fig 16 B].
{image:48}
{image:49}
Nonseminomatous Germ Cell Tumor (NSGCT)
These include yolk sac tumor, embryonal cellcarcinoma, teratoma, and choriocarcinoma and mixed germ cell tumors. Mixed germcell tumor is the most common NSGCT. Sonographicaly, NSGCT tend to be more heterogeneous in echotexture, with both solid and cystic components and echogenic foci, with irregular or ill-defined margins [Fig 17A, 17B, 17C]. The echogenic foci can be due to calcification, hemorrhage, or fibrosis.
{image:50}
{image:51}
{image:52}
Lymphoma
It is the most common testicular tumor after the age of 60, with bilateral involvement in 40% of patients. Most primary testicular lymphomas are non-Hodgkin lymphomas. However, secondary involvement is much more common than a primary neoplasm. At US, multiple focal hypoechoic masses may be present or diffuse enlargement may occur [Fig 18A, 18B]. Color Doppler US shows increased vascularity regardless of the size of the lesion [31].
{image:53}
{image:54}
Benign Testicular Lesions
Most intratesticular tumors are malignant; majority of intratesticular cystic lesions are benign, which can present as painless testicular masses, correct diagnosis of these can prevent unnecessary surgical exploration.
Cysts of the tunica albuginea
They can be unilocular or multilocular of size 2–5 mm. They are often detected when a patient presents with a palpable mass. [32] The etiology is unknown, but these cysts are believed to be mesothelial in origin. These cysts sometimes calcify, which casts an acoustic shadow [Fig 19].
{image:55}
Simple cysts
Simple cysts are often incidentally detected in men around 40 years of age, usually solitary; vary in size from 2 mm to 2 cm. At US, they appear as an anechoic, without a perceptible wall and with increased through-transmission [Fig 20].
{image:56}
Tubular ectasia of rete testis
Tubular ectasia of the retetestis is a benign condition, occurs in men older than 55 years and is frequently bilateral. Findings of cystic dilatation in or adjacent to the mediastinum testis are characteristics of tubular ectasia and aid in distinguishing it from malignant cystic testicular tumors, which can occur anywhere in the testicular parenchyma. The US appearance is of fluid-filled tubular structures [Fig 21].
{image:57}
Intratesticular abscess
An abscess is usually secondary to epididymo-orchitis, but other causes of intratesticular abscess include mumps, trauma, and testicular infarction. The US features include intra testicular hypoechoic, shaggy irregular walls, unifocal or multifocal, with low-level internal echoes, and, on colour Doppler, shows hyper vascular margins [Fig 22A, 22B].
{image:58}
{image:59}
Testicular Calcification
Testicular microlithiasis
Testicular microlithiasis (TM) is usually discovered incidentally at US. The typical US appearance of TM is of multiple nonshadowing echogenic foci measuring 2–3 mm and randomly scattered throughout the testicular parenchyma [36] [37] .TM is 2 types [38], depending on the number of echogenic foci per image. With 5 or more echogenic foci on a single image called as classic TM [Figure 23A], with fewer than 5 echogenic foci is called as limited TM [Figure 23B]. It is recommended for annual US follow-up for at least several years after the diagnosis, since associations with testicular neoplasia has reported. [39]
{image:60}
{image:61}
Macrocalcifications
Macrocalcifications can be intra- or extra testicular. Calcifications in the epididymis can occur secondary to inflammatory conditions such as tuberculosis or trauma [Fig.24A].
{image:62}
Scrotoliths (scrotal pearls)
These are calcified bodies caused by torsion of appendix testis or appendix epididymus, lying between the membranes of the tunica vaginalis that have no clinical importance. [20] [40] US shows solitary, round hyper echoic area and measure up to 1 cm in diameter, producing a discrete acoustic shadow [Figure 24B].
{image:63}
Testicular Trauma
Testicular trauma is the third most common cause of acute scrotal pain. Sporting activities account for more than half of all cases of testicular injury. More than 80% of ruptured testes can be salvaged, if surgical repair is performed within 72 hours of testicular injury [42]. US reliably depicts tunica albuginea rupture, intra- and extra testicular hematomas, and testicular contusions there fore useful in triage of patients for medical or surgical management. US findings in testicular rupture include an interruption of the tunica albuginea, a heterogeneous testis with irregular poorly defined borders, scrotal wall thickening, and a large hematocele [Fig 25A]. Color and power Doppler US can demonstrate disruption in the normal capsular blood flow of the tunica vasculosa. Hematocele is a blood collection within the leaves of the tunica vaginalis. At US, an acute hematocele is echogenic, whereas an older hematocele appears as a fluid collection with low-level echogenicity [Fig 25B], fluid-fluid level, or septations.
{image:64}
{image:65}
Scrotal Filariasis
The “Filarial dance sign” (FDS) [43] is described on HRUS shown as linear echogenic structures with persistent, random, almost tireless twirling movements of live adult filarial worms in the lymphatic vessels [Fig 26A]. Pulse Wave Doppler reveals worm nests, in enlarged lymphatic vessels by the characteristic pattern of irregular worm movements, in color Doppler visualized in form of an irregular red color signal [Fig 26B].US shows dilatation in the lymphatic vessels, early and advanced stages of hydrocoele, and the number of worm nests over time. On follow-up US, after the treatment with DEC, Complete absence of worm movements was taken as a positive response[44] [Fig 26C].
{image:66}
{image:67}
{image:68}
Conclusion
In conclusion, the high frequency and color Doppler US is an inexpensive and non-irradiating, non invasive, widely available, easy for follow up and an accurate imaging technique for diagnosis of a variety of scrotal lesions. High-frequency US in addition to color Doppler is a modality of choice to differentiate testicular torsion from inflammatory conditions and diagnosis of scrotal trauma. Gray-scale and color Doppler US accurately differentiates testicular from extra testicular from extra testicular lesions and correctly identify benign intratesticular cystic lesions and differentiate them from malignant testicular lesions, with the goal being to prevent unnecessary surgical intervention. Accurate localization of the testis is possible in high inguino scrotal region and diagnosis of varicoceles can be done in infertility. Therefore high resolution sonography and colour doppler is a first imaging modality in all spectrum of scrotal pathologies.