Giant Bathing Trunk Naevus with Multiple Congenital Melanocytic Naevi
S Sharma, N Sharma, V Sharma
Keywords
congenital nevomelanocytic nevus cnn, giant congenital melanocytic nevi gcmn, malignancy
Citation
S Sharma, N Sharma, V Sharma. Giant Bathing Trunk Naevus with Multiple Congenital Melanocytic Naevi. The Internet Journal of Pediatrics and Neonatology. 2012 Volume 14 Number 1.
Abstract
Congenital nevi are hyperpigmented macular lesions that are derivatives of the melanoblasts. They occur in less than 1% of the neonates in any site of the body. The giant congenital nevus is greater than 20 cm in size, pigmented and often hairy. Between 4% and 6% of these lesions will develop into a malignant melanoma. Since approximately 50% of the melanomas develop by the age of two, and 80% by the age of seven, early removal is recommended. However, their large size poses a great treatment challenge. The objective of this paper is to present a unique case of giant nevi along with a review of the literature .
Introduction
Nevus, a Latin word Knee-vus meaning “birthmark,” or “mole,” is a general term for a congenital mark on the skin. Congenital melanocytic naevi are brown or black moles which are present at birth or which develop in the first year of life [1] . They are formed by overgrowth of the melanocytes. A giant congenital melanocytic nevus (GCMN), giant hairy nevus or nevocellular nevus represents a special group of melanocytic lesions that generally covers large areas of the body and have a potential risk for developing malignant melanoma [2,3]. The lesion is variously called bathing trunk, cape, vest, coat¬sleeve or stocking naevus depending on regional distribution [4]. The development of new surgical techniques and the expansion of laser technology will possibly provide new treatment options in the future. Emphasis should be placed on aesthetics and function, because the excision based only on oncological anticipation is no longer valid. The present paper emphasizes the importance of recognizing the worrisome deep extension of pigmentation seen in the GCMN and raises considerations toward other treatment options.
Case Report
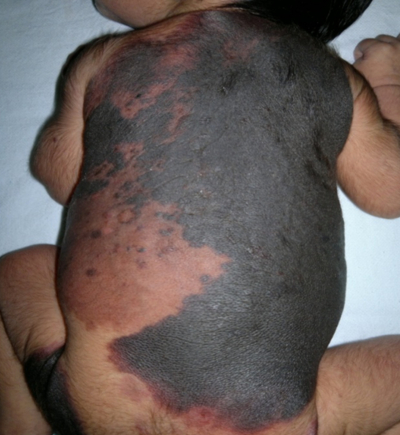
A 5 days old female neonate born out of non-consanguineous marriage by normal vaginal delivery at term to a primigravida with uneventful antenatal history presented with an extensive pigmented patch over the body since birth. Physical examination revealed an extensive pigmented patch covering 40% of the skin surface covering the back, neck, buttocks and left thigh (Fig.1, Fig2) .
Multiple pigmented satellite lesions were also present over the extremities. Tufts of coarse and lusterless hair were scattered over the lesion in right scapular region which were1-2 cm in size.(Fig 3)The lesion was black in colour with convolutions on the back. There were also present small multiple pigmented satellite lesions of varying diameters (1cm to 3 cm), scattered all over the body . There were no other associated congenital anomalies. Fundus examination, X ray spine, CT head and spine and ultrasound abdomen were all normal. The biopsy of the patient was taken and the histopathological findings were consistent with congenital melanocytic naevus. There were nest & cords of naevus cells filling the dermis and extending into the subcutaneous tissue (Fig 4). The dermis consisted entirely of heavily pigmented naevus cells containing melanin. No junctional activity or evidence of malignant transformation was seen. The rest of the examination was normal. There was a negative family history of similar lesions. The baby was discharged home after three days and she is under regular follow up in our clinic.
Discussion
The congenital melanocytic nevi are pigmented cutaneous lesions formed by a combination of epidermally and dermally derived naevus cells occurring in about one per cent of the newborns. It has it's color from the melanin pigment of nevomelanocytes. Nevomelanocytes, derivatives of melanoblasts, compose the cellular format of the neoplasm [5]. They are classified according to their size as small (<1.5 cm), medium (1.5 - 19.9 cm) and large or giant nevi (>20 cm). Giant congenital nevi or giant hairy nevi have an irregular margin often with a verrucous surface. Their color is typically dark brown to black and 95% of them have dark, coarse surface hair. Satellite lesions are often present beyond the periphery of the main lesion and may be scattered over the entire skin surface. Around the age of 10 the giant nevus becomes more elevated, verrucous, and hyperkeratotic and the surface hair thicker [6]. Clinically, they are characterized by large black patches presenting at birth commonly over the back and thigh area, with smaller s satellite lesions. Giant hairy nevi on the scalp and neck may be associated with leptomeningealmelanocytosis and neurologic disorders that include neurofibromatosis, epilepsy or focal neurologic abnormalities. Lesions over the vertebral column may be associated with spina bifida or meningomyelocele sometimes complicated by hydrocephalus and malignant melanoma.
The incidence for small nevi is 1 in 100 births; for medium nevi 6 in 1000 births; a GCMN larger than 20 cm in diameter occur in 1 per 500,000 newborns[7,8]. An equal prevalence exists in males and females. Congenital nevomelanocytic nevus (CNN) appear in all races, but, paradoxically, the frequency of small CNN is slightly higher in blacks who are at lower risk of developing melanoma than white [9]. Autosomal dominant inheritance with incomplete penetrance or multifactorial determination occurs in families with small CNN [10]. GCMN are thought to be caused by spontaneous mutations or other events during fetal development, but in some families, the frequent appearance of these lesions suggest that they may be genetically inherited. The genetic basis of these lesions is not known. Findings of a culture of melanocytes from such a lesion showed chromosome rearrangements involving 1p,12q, and 19p. The skin and nerves of a fetus develop from the same primary cells of the body, which are called neuroectodermal cells, between the 8th and 24th week of pregnancy. Melanoblasts migrate from the neural crest between weeks 8 and 10 of gestation [10]. Researchers think that a body protein called HGF/SF (hepatocyte growth factor/scatter factor) seems to be responsible for encouraging these neuroectodermal cells to develop, migrate, and “scatter.” It seems that either too much or wrong type of this body protein HGF/SF in some, not all, of our cells , develop extra pigment and abnormal skin cells called nevus cells. These cells “scatter” around, so we have nevi “scattered” all over us. They may be associated with other birth defects. That may be why some of us develop nerve symptoms like hydrocephalus, melanoma, and neurocutaneous melanosis [11]. Histologically, nevi are transformed melanocytes, which are normally highly dendritic cells interspersed among basal keratinocytes.
Malignant potential of these lesions with the incidence ranging from 2-41% [12].Also, about 40% of the malignant melanomas in children arise within these GCMN [13]. The potential for large congenital nevi to become malignant is significant and is an important consideration in the treatment and management of this entity. The size of the lesion correlates with the potential for malignant transformation. Malignancy should be suspected with focal growth, pain, bleeding, ulceration, significant pigmentary change, or pruritus. The risk of malignant melanoma in patients with small to medium ones range from 2.6% to 4.9%, while for giant nevi the risk is felt to be approximately 6% [14]. Malignant change of small nevi usually occurs after puberty, whereas 60% of all malignant melanomas arising from giant nevi will develop in the first decade of life.
The impact of GCMN is greater because of the considerable cosmetic disfiguration which is very distressing to the parents along with its higher malignant potential. The risk of malignancy is also increased by the presence of larger nevi (greater than 50 cm), axial location such as trunk, head and neck, the presence of multiple satellite lesions, and the existence of nodules, dark patches, junctional activity, deep dermal neurogenic element or a blue nevus component [9,15,16,17,18,19,20]. Radiographic imaging, including MRI, is warranted to evaluate melanocytic depositions in the CNS. The baseline MRI should be obtained when the patient is aged 4-6 months. Serial MRIs are frequently required in patients with meningeal melanocytosis [6].
The management and treatment of patients with GCMN remains controversial. No absolute guidelines can be recommended. Management is individualized and centered around: cosmetic appearance, risk of neoplastic proliferation, and the psychological impact on the patient and family [6,7,9,12,21,22]. Aesthetic considerations are important. It is impractical to prophylactically excise all non–giant congenital nevi, yearly examination for the first 3 years of life is recommended, with reassessment every 2 to 5 years afterwards depending on the confidence of the parents to monitor the lesions. Biopsy specimens are obtained from lesions that undergo suspicious alteration.
Surgical treatment of GCMN is addressed at age 6 months. Procedures used in surgical treatment include serial excision and reconstruction with skin grafting, tissue expansion, local rotation flaps, and free tissue transfer. Due to the depth of some lesions, especially if the leptomeninges are involved, excision may not eliminate the risk for developing melanoma [16]. Adjunctive treatment options include chemical peels, dermabrasion, and laser surgeries. Dermabrasion has also been successfully employed with gratifying cosmetic results, but leaving behind of nevus cells in the deep dermis has been a serious objection to the procedure. The carbon dioxide laser, the Er : YAG and the Q-switched ruby laser have all been recently used for resurfacing and to selectively treat deep pigmentation[23,24,25,26].
More confusion is generated due to the published wide range of malignant transformation risk from less than 1% to 31%. While most cases of melanoma arising within the GCMN occur before puberty (14), with a reported incidence of melanoma of 8.52%, the life-risk is in the range of 2.3%.
Given that the risk of malignant disease is lower than previously reported, and that even after surgical treatment the risk does not disappear, we believe that when the decision is made to proceed with some form of treatment, greater attention should be paid to achieving a more functional and aesthetic result, because the excision based only on oncological anticipation is no longer acceptable.
The case report of GCMN is being presented because of its rarity and evolving methods of management.