Adenomyoepithelioma A Rare Breast Tumor: Case Studies With Review Of The Literature
V Satyanarayana, S Gole
Keywords
adenomyoepithelioma, apocrine metaplasia, cystic degeneration., myoepithelial cell
Citation
V Satyanarayana, S Gole. Adenomyoepithelioma A Rare Breast Tumor: Case Studies With Review Of The Literature. The Internet Journal of Pathology. 2012 Volume 13 Number 2.
Abstract
Introduction
Benign adenomyoepithelioma of the breast is a rare tumor characterized by biphasic proliferation of both an inner layer of epithelial cells and a prominent peripheral layer of myoepithelial cells. It was first described by Hamperl[1] in 1970; more than 60 cases have been reported since then. All cases except 2 occurred in females.[2,3] Benign adenomyoepitheliomas outnumber their malignant counterparts by far. Malignancy may arise either through malignant transformation of 1 of the 2 cellular components or through malignant transformation of both.[4] The difficult differential diagnosis, potential for recurrence and malignant evolution of this lesion merit a careful approach.
Materials and methods
In two cases of breast lump, FNAC was done and stained with H & E and Papanicolaou stains. The formalin-fixed tissue sections were stained with hematoxylin and eosin, and in one case immunohistochemistry was performed with smooth muscle actin (SMA).
Figure 1
Figure 2
Modified radical mastectomy was done and sent for histopathology. The specimen of breast along with axillary pad of fat measured 18 x 17 x 6 cm. There was a well circumscribed grey white solid mass measuring 3.5 x 3 x 3 cm. The mass was situated 0.2 cm beneath the nipple and 0.5 cm away from the lower resected margin. Nine lymph nodes were dissected from the axillary pad of fat. The lymph nodes measured 0.2-2 cm in diameter. Histopathological sections from the breast mass showed a well delineated tumor composed of predominantly round to irregular tubular structures lined by basal myoepithelial cells with focal cell proliferation having clear cytoplasm (Figure 3).
Figure 3
The luminal epithelial lining was cuboidal to columnar with round to oval vesicular nuclei having eosinophilic cytoplasm and apocrine snouts. The intervening stroma was edematous (Figure 4, 5).
Figure 4
Figure 5
No significant mitotic activity observed. There was no local invasion and the adjacent breast parenchyma revealed the normal breast lobules along with the intervening stroma. Reactive changes were observed in all 9 resected lymph nodes. The histopathological diagnosis was adenomyeoepithelioma – breast (right), tubular type.
Figure 6
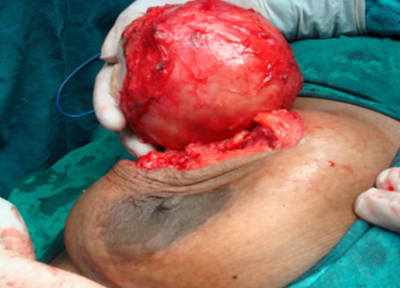
Peroperatively it was found to be a cystic swelling occupying whole of the breast (Figure 7).
The mass was excised and sent for histopathology. The smooth, well encapsulated, grey white to yellow cystic mass measured 10 x 9 x 5 cm. Cystic cavity was filled with yellow pultaceous and calcified material (Figure 8, 9).
Figure 8
Histopathologically, sections from the cyst wall showed a compressed tumor tissue exhibiting proliferating glandular structures of varying size with intervening fibrocollagenous stroma. The glands with basal lamina were lined by basal cuboidal cells with clear cytoplasm and luminal columnar cells with apocrine metaplastic change. The cells revealed benign nuclear features (Figures 10-13).
Figure 10
Figure 11
Figure 12
Figure 13
The inner fibrous wall of the cystic mass showed foamy degenerative cells and the cyst lumen was filled with acellular proteinaceous material (Figure 14).
Figure 14
Upon immunostaining with smooth muscle actin (SMA), the myoepithelial cells showed strong positivity (Figure 15, 16).
Figure 15
Figure 16
It was diagnosed as adenomyoepithelioma with apocrine metaplasia and cystic degeneration – breast (left), tubular type.
Results
Two cases of breast lump were managed surgically. Both the cases were females with the mean age being 27 years. Both cases were of adenomyoepithelioma (tubular type) with one case showing apocrine metaplasia and cystic change.
Discussion
Myoepithelial cells are a normal component of the breast tissue and are located between the luminal cells and the basal lamina, of the mammary duct and lobular system. The most common lesion affecting these cells is sclerosal adenosis.[5] Their presence in neoplastic lesions has been considered a hallmark of benignity. Recently, however, breast neoplasms have been described that are entirely or partially composed of myoepithelial cells. Neoplasms of purely myoepithelial origin have been called myoepitheliomas
Breast adenomyoepithelioma arises from a mixture of epithelial and myoepithelial cells and is a rare, myoepithelial cell-rich neoplasm. It is closely related to adenomyoepithelial (apocrine) adenosis and sometimes also considered an uncommon variant of intraductal papilloma.[6,7,8]
The exact etiology of breast adenomyoepithelioma is still obscure. All cases have been sporadic and no familial aggregation has been observed. Kiaer et al.[9] reported a case of sequential changes from adenomyoepithelial adenosis into adenomyoepithelioma which eventually became low grade malignant adenomyoepithelioma during the course of 18 years. From this observation, Choi et al.[10] proposed that adenomyoepithelioma was derived from a myoepithelial, long-standing, underlying breast disease such as adenosis and fibroadenoma. The association of adenomyoepithelioma with adenosis has been documented.[4]
The lesion is usually well circumscribed and consists of myoepithelial cells admixed with luminal elements: its histology depends on the relative proportion of luminal and myoepithelial cells, the existence of any papillary component, and the extent of fibrosis. Histology varies from a tubular pattern to spindle cell or even clear cell. Focal apocrine, squamous, mucinous, sebaceous or even chondroid metaplasia may be encountered.[6] Distinctive features of myoepithelial cells in adenomyoepitheliomas include their frequent, clear, abundant cytoplasm and their peripheral location in individual acini. They may also be spindled, resembling the cells of leiomyomata, or be eosinophilic. Epithelial cells by contrast have eosinophilic cytoplasm and a central location in the acini. They may display apocrine metaplasia[11] as was seen in our case 2. Epithelial lumina may be reduced to slit-like spaces by the expansion of myoepithelial cells.
Tavassoli[11] proposed a classification system of myoepithelial lesions of the breast. These lesions were divided into: myoepitheliosis, adenomyoepithelioma and malignant myoepithelioma. Tavassoli[11] classified adenomyoepitheliomas as spindle cell, lobulated, and tubular (or adenosis) types with carcinoma arising in adenomyoepithelioma. The most common pattern is the tubular type with features characterized by proliferation of glandular cells and surrounding myoepithelial cells of abundant clear cytoplasm, as was seen in both our cases.
Differential diagnosis of adenomyoepithelioma includes sclerosing adenosis, fibroadenoma, tubular adenoma, and pleomorphic adenoma. Tubular adenoma, sclerosing adenosis, and fibroadenoma have less prominent proliferative features compared with adenomyoepithelioma. Pleomorphic adenoma usually has prominent areas of chondroid and osseous differentiation. Proliferative myoepithelial cells with clear cytoplasm may mimic malignancy in an intraoperative frozen section; therefore, it is difficult to make an accurate diagnosis. Accurate diagnosis can be difficult based solely on radiological observation; therefore, histological examination results are required to make the precise diagnosis.[12]
Benign adenomyoepitheliomas have mammographic findings, which are often suggestive of a benign lesion. The sonographic appearance may vary from a cystic to a solid mass.[5] In our case 1, ultrasound findings were suggestive of duct ectasia.
Fine needle aspiration cytology (FNAC) is usually a good choice for diagnosis of breast lumps: a diagnostic accuracy of 96.9% and positive predictive value of 98.4% have been reported. Cytologically, adenomyoepithelioma usually shows moderate to high cellularity.[5] In our case 1, FNAC report was proliferative breast disease with atypia suggestive of duct cell carcinoma, and in case 2, FNAC was suggestive of fibroadenoma.
In the cytological differential diagnosis, adenomyoepithelioma may mimic other myoepithelial/stromal cell-rich lesions: the classical example is phyllodes tumor. In malignant phyllodes tumors, there is an increase in cellular pleomorphism and mitotic activity of the spindle cells. The rare, adenoid cystic carcinoma of the breast represents another biphasic tumor. FNAC of adenoid cystic carcinoma often yields the characteristic three-dimensional tubular or cribriform structures associated with amorphous hyaline globules. Adenomyoepithelioma sometimes mimics myofibroblastoma or even metaplastic carcinoma on FNAC. In adenomyoepithelioma with a predominant apocrine cell population, the distinction from apocrine carcinoma is difficult. A spectrum of findings is seen on the biopsies of adenomyoepithelioma of the breast and may result in difficult differential diagnosis with other lesions.[5]
Myoepithelial cells can be identified histologically, immunohistochemically, and by electron microscopy. Histologically, myoepithelial cells may be difficult to recognize. Lack of familiarity with their range of appearances may at least in part be responsible for the paucity of recognized cases.[11] Immunostaining for smooth muscle actin and calponin can support the myoepithelial differentiation as no reactivity is noted in epithelial cells. S-100 protein has been used as a marker of myoepithelial cells; however, its reaction is not consistent and may be expressed in both myoepithelial cells and epithelial cells and can also be seen in invasive breast carcinomas. Therefore it is not a reliable marker for myoepithelial cells in adenomyoepitheliomas.[12] Tamai reported a case of myoepithelioma in which the clear myoepithelial cells showed no reaction to actin. Myoepithelial cells stain with actin to varying degrees, depending on their level of differentiation. According to Bassler and Katzer, cells stained with antibodies against S100 and cytokeratins ,but not with antibodies against smooth muscle actin, express incomplete features of the fully differentiated myoepithelial cells and can be considered transitional, intermediate, or precursor cells.[4] Our case 2 showed strong positivity for smooth muscle actin. Adenomyoepithelioma also shows strong positivity for keratins CAM5.2 and for EMA in the epithelial component, whereas the myoepithelial cells express several specific markers, including muscle- specific actin and myosin, calponin, p63, CD10 and S-100 protein.[13]
Malignant transformation in the form of ductal carcinoma, malignant myoepithelioma or leiomyosarcoma is frequent and may result in local recurrence or even distant metastasis: radical surgery is important to ensure good local control of disease. A correct preoperative diagnosis is also important for surgical planning. To date, only 13 cases of adenomyoepithelioma with detailed fine needle aspiration biopsy findings have been described in the literature.[5]
Adenomyoepithelioma is currently considered an indolent breast neoplasm but with a potential for local recurrence and distant metastasis, this is especially true if malignant transformation occurs within the original mass.[5] Malignant neoplasms arising in adenomyoepitheliomas have been described in many patterns and have been subclassified as undifferentiated, myoepithelial, or epithelial. Malignant adenomyoepithelioma in which both cellular components underwent malignant transformation is exceptionally rare, and only 12 cases have been reported.[4]
Malignant adenomyoepithelioma of the breast is a rare lesion characterized by malignant proliferation of epithelial and myoepithelial cells that show characteristic histologic and immunohistochemical features. Most of the cases demonstrate malignant transformation of only one cellular component, either epithelial or myoepithelial, though more often epithelial.[4]
A focal infiltrative growth pattern increases with successive local recurrences. Complete surgical excision with adequate margins is required, especially in view of tendency for local recurrence and malignant transformation.[8,14] The malignant type of adenomyoepithelioma can show nodal and distant metastases. Loose et al. have proposed three malignancy predictors, including high mitotic rate, cytologic atypia, and infiltrative peripheral border for benign adenomyoepitheliomas.[15]
In the therapeutic approach to this disease, all findings and results should be assessed together to decide ultimate surgical planning so as to avoid unnecessary wide surgical resections and complications, but also to ensure a radical therapy for the patient.[5] In our case 1, clinical examination and the FNAC, were strongly suggestive for breast malignancy so modified radical mastectomy was done. The potential for the adenomyoepithelioma to recur locally and, sometimes, to give distant metastasis could justify resorting to breast surgery.[5]
The association of benign adenomyoepithelioma with a malignant lesion has been reported. The malignant lesions reported to arise from a benign adenomyoepithelioma, however, were all carcinomas. The evolution of malignant adenomyoepithelioma seems to begin with adenosis, with or without myoepithelial hyperplasia, proceed to benign adenomyoepithelioma, and end in a malignant tumor that still may contain residues of its precursor lesion.[4]
Biphasic malignant adenomyoepithelioma is a rare neoplasm, so far only reported in women who range in age from 26 to 76 years. Tumor sizes range from 1 to 15 cm. Metastasis may occur, hematogenous rather than lymphatic, and so far has been seen in tumors 2 cm in diameter or larger.[4]
Not much is known about the natural history of biphasic malignant adenomyoepitheliomas. Of the 11 published cases, follow-up was reported in 4 cases and ranged from 12 to 64 months. Metastasis was described in 4 cases. Trojani et al. reported a case of malignant adenomyoepithelioma with lung metastasis. One of the 2 cases reported by Loose et al.[15] also showed lung and brain metastases. The primary tumors were 2 and 3.5 cm, respectively. Metastatic tumors were composed of both epithelial and myoepithelial cells. Simpson et al. described a unique case of malignant adenomyoepithelioma in which a carcinoma developed from the epithelial component and the tumor contained a metaplastic carcinoma with osteogenic and undifferentiated areas. In this case, the tumor recurred twice, metastasized, and caused death after 39 months. In these cases, the presence of distant metastasis was a clear sign of malignancy. Rasbridge and Millis reported 7 cases of adenomyoepithelioma, 5 of which were classified as malignant based on cytologic and architectural criteria. In one case, the patient died as a result of clinically suspected cerebral metastasis.[4]
However, as Loose et al.[15] suggested, the term malignant adenomyoepithelioma can also be applied to tumors that have not yet metastasized but are locally invasive and show high mitotic activity and marked cytologic atypia. In our cases, there was absence of local invasion, high mitotic activity, and cytologic atypia indicating the benign nature of the lesions. Although case 1 presented with axillary lymphadenopathy, on histopathology only reactive changes were observed.
Conclusion
Prognosis of patients with benign adenomyoepitheliomas of the breast is usually good, but it has the potential for local recurrence, especially in the tubular and lobulated variants. Total surgical excision with an adequate margin of uninvolved breast tissue is therefore recommended. Failure to achieve a free resection margin may result in local recurrence or rarely, malignant transformation.