Pneumococcal pneumonia complicates presentation of pulmonary tuberculosis and pseudomembranous candidiasis, predictive of unknown HIV infection in Ekpoma Nigeria
J Ihongbe, E Agwu, N Inyang
Keywords
ekpoma, hiv, pneumococcal pneumonia, pseudomembranous candidiasis, pulmonary tuberculosis
Citation
J Ihongbe, E Agwu, N Inyang. Pneumococcal pneumonia complicates presentation of pulmonary tuberculosis and pseudomembranous candidiasis, predictive of unknown HIV infection in Ekpoma Nigeria. The Internet Journal of Microbiology. 2007 Volume 5 Number 2.
Abstract
This study outlined how pneumococcal pneumonia complicated the clinical presentations of pulmonary tuberculosis and showed the predictive value of Pseudomembranous candidiasis (PC) in detection of unknown Human immunodeficiency virus (HIV) infection in Ekpoma. Out of 510 sputum samples analyzed, bacteria and fungi
Introduction
In most developing countries like Nigeria, the problem of inability to find out the exact cause of infection before commencement of treatment is high especially in remote rural areas. This could be due to lack of good health facilities with standard diagnostic equipments and qualified laboratory staff. This has promoted over-dependence on presumptive diagnosis and treatment of infectious agents based sorely on clinical findings. Consequently many infectious agents are either misdiagnosed or undiagnosed.
Preliminary survey reveal that increasing evidences of recurrent lower respiratory tract (LRT) infection among patients attending tuberculosis clinics in Ekpoma suggest other microbial etiology in addition to
This study which is entirely laboratory based, was therefore designed to show the role of underlying pneumococcal pneumonia in complicating LRT (tuberculosis) infections among patients attending tuberculosis clinics in Ekpoma Nigeria and to re-emphasize the significance of laboratory investigations in the management and control of infections.
Materials and Methods
Sampling Area
Ekpoma and its environs in Esan West Local Government Area of Edo State, Nigeria were the main study areas. It is a University town situated 120km north of Benin, the capital city of Edo State Nigeria. It has few private clinics with no specialist or referral hospital. Four private clinics and one General Hospital, all in Ekpoma, served as the major sites for sample collection. Informed consent of the patients were sought and obtained in writing.
Inclusion criteria
There were respiratory symptoms suggestive of pulmonary infections which were used as criteria for inclusion of patients in this investigation. The characteristics of patients enrolled in this investigation included: prolonged chronic productive cough, constant weight lost, fever, loss of appetite, weakness, night sweats, malaise, pulmonary inflammation and necrosis, peripheral adenopathy, general chest pain and difficulty in breathing. Pseudomembranous candidiasis was detected on visual inspection of the oral cavity in the Laboratory. There was no chest X-ray for patients to have guided the use of radiographic resolution in the diagnosis of lobar pneumonia and no facilities for cultural isolation of
Selection of suitable samples for analysis
Smears of the purulent parts of the specimens were made on a glass slide for Gram-staining before transportation to Search-Light Medical Diagnostic Center Ekpoma for further processing. Direct examination of samples by the Grams staining technique was adopted in selecting ideal samples for culture (Agwu
Staining and cultivation of samples for Microbial etiology
The Ziehl Nielsen technique of staining for
Serological detection of HIV among patients with oral thrush
Sera of the patients whose sputum samples yielded significant growth of
Results
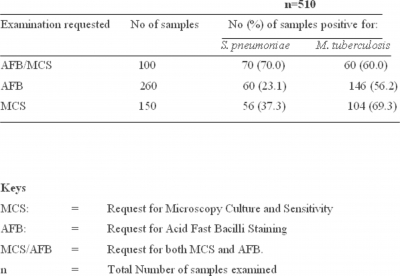
The result of this survey reveals that out of the 510 sputum samples analyzed, 225 (44.1%) were co-infected with both
Oral lesions (pseudomembranous candidiasis) were also observed in the oral mucosa of the patients whose samples yielded significant growth of
n= total number of patients examined
Figure 2
Discussion
Lack of adequate health care facilities in Ekpoma is a major health concern for Esan West Local Government (Edo State Nigeria) and the inhabitants of Ekpoma community. Presumptive disease diagnosis and empirical treatment are therefore on the increase in the private health sector predominant in Ekpoma. The co-infection of
Table II shows undiagnosed but detected infections of pneumococcal pneumonia and pulmonary tuberculosis. In brief, out of 260 patients clinically diagnosed with pulmonary tuberculosis, 60 (23.1%) harbored
Furthermore, most of the clinical diagnosis made based on the observable signs and symptoms of lower respiratory tract (LRT) infections were inaccurate. It is therefore important for rural health-care providers to note that significant percentage of empirical diagnoses made based on clinical manifestation of LRT infections and other diseases may be unreliable as the sole bases for treatment. This re-emphasizes the non-specific nature of most clinical manifestations of lower respiratory track infection and portrays the danger of relying only on clinical findings as sole basis for disease management without necessary laboratory investigations. For improved and effective empirical management of pulmonary tuberculosis and
The observed diagnosis of pulmonary tuberculosis and subsequent request for detection of acid and alcohol fast bacilli while leaving other underlying infections such as pneumococcal pneumonia may be as a result of bizarre clinical presentation or asymptomatic infection. This point is supported by our former report that about 95% of tuberculosis infections may be asymptomatic and 70% of
The clinical relevance of the
Available reports from different African geographical regions differ on the positive predictive values of the different clinical presentations of Candida infection for underlying HIV disease. The presence of any form of Candidosis was significantly associated with HIV infection in subjects, from various clinical settings, in Tanzania (Shiodt
Pneumococcal pneumonia complicated the diagnosis of pulmonary tuberculosis in Ekpoma due to lack of modern diagnostic facility for full cultural and radiographic resolution of the two infections. On the other hand, pseudomembranous candidiasis was highly predictive (79.2%) of new HIV infection among the studied population. Full laboratory investigation remains invaluable in the management, prevention, and control of infectious agents, especially in conditions where underlying etiologic agents of diseases may pose a public health problem. The role of the laboratory is therefore very important in the diagnosis of mixed, dual, or double infections. Further study would be needed to establish the predictive value of other oral lesions so as to develop simple diagnostic algorithm to help healthcare providers in detection of new HIV disease. Nigerian Government is hereby advised to improve the condition of services at primary health centers, make the services affordable and extend such services to more remote villages where the ordinary citizens can access it.
Correspondence to
Agwu Ezera Department of Medical Microbiology, School of Healthsciences, Kampala International University, 56 Mbarara-Fort Portal Road, Ishaka, Box 71, Bushenyi, Uganda. E-mail: agwuezera@yahoo.com or kingezera@hotmail.com Phone: +256782101486; +256703129679