Antimicrobial susceptibility of Salmonella typhi and Staphylococcus aureus isolates and the effect of some media on susceptibility testing results
E Donkor, T Nortey, J Opintan, N Dayie, M Akyeh
Keywords
and tryptone soy agars, kirby bauer, mueller-hinton, nutrient
Citation
E Donkor, T Nortey, J Opintan, N Dayie, M Akyeh. Antimicrobial susceptibility of Salmonella typhi and Staphylococcus aureus isolates and the effect of some media on susceptibility testing results. The Internet Journal of Microbiology. 2007 Volume 4 Number 2.
Abstract
(i) to determine the antibiogram of
The Kirby Bauer method was used to evaluate the susceptibility of 30 isolates each of
The prevalence of multiple drug resistance as determined on Mueller-Hinton agar was 83.3% for
The high resistance rates observed for the organisms to some of the drugs underscore the need for susceptibility testing. However, the use of Nutrient and Tryptone Soy agars for the Kirby Bauer method as practiced by some laboratories in Ghana is discouraged.
Introduction
Antibiotic-resistant organisms lead to increased hospitalisations, health costs, and mortality. Antimicrobial drug resistance has therefore become an important public health concern associated with serious consequences for the treatment of infections (1,2). The phenomenon has been attributed to the misuse of antimicrobial drugs which provide selective pressure favouring the emergence of resistant strains. To contain the problem of antimicrobial resistance, the World Health Organization has provided some interventions, one of which includes effective surveillance of antimicrobial resistance among common pathogens. In Ghana,
A number of tests are available for susceptibility testing and hence surveillance of antimicrobial resistance. However, the most widely used method is the disc diffusion test, which comprises mainly the Kirby-Bauer and the Stokes methods (6). The two methods differ in their standardisation, reading, and control. While the Stokes method permits the use of any media, the Kirby-Bauer method is limited to Mueller-Hinton medium. Additionally, the control and the test organisms for the Stokes method are tested on the same plate whilst for the Kirby-Bauer method, they are carried out on different plates. In Ghana, the Kirby-Bauer method is the main susceptibility method employed in microbiology laboratories (3). A survey showed that some laboratories use other media particularly, nutrient and tryptone soy agars in place of Mueller-Hinton, the recommended medium for the Kirby-Bauer method (3). At present, there has been no study yet in the country to document the quantitative effect of these non standard media on quality of susceptibility testing results. Since the use of inappropriate media could affect effective surveillance of resistance and ultimately result in ineffective therapy, there is the need for an investigation to help address this problem.
The objectives of the study were to (i) determine the antibiogram of
Materials And Methods
This prospective study was carried out from June to September, 2006 at the Microbiology Laboratory of the Korle Teaching Hospital located in Accra, Ghana. The hospital is the largest of the three tertiary hospitals in Ghana, and a major referral centre not only for Ghana, but other West African Countries. The Microbiology Laboratory receives high numbers of clinical specimens from patients, and the isolation rate of bacteria from specimens is about 2000 per year (3). Some of the common organisms isolated from clinical specimens in this laboratory include,
A total of thirty isolates each of
The laboratory analyses results were analysed in STATA 7.0 to address the objectives of the study. Zone diameters of susceptibility testing results were categorised as sensitive or resistant based on the NCCLS breakpoint criteria (9). Following this, comparisms of susceptibility testing results on Mueller-Hinton agar were compared with those of nutrient agar as well as tryptone soy agar. The prevalence rates of drug resistance including multiple resistance (resistance to three or more drugs) of
Results
Drug Resistance of Salmonella typhi and Staphylococcus aureus Isolates
The prevalence rates of resistance of
Comparism of Susceptibility Testing Results on Different Media
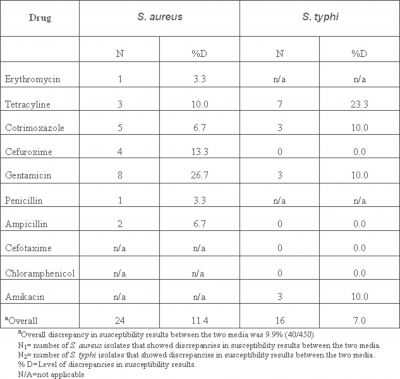
Tables 1 and 2 report on the discrepancies of susceptibility testing results between Mueller-Hinton agar and each of Nutrient and Tryptone Soy agars. At 5% level, significant discrepancies were observed in susceptibility testing results between Mueller-Hinton agar and each of Nutrient and Tryptone Soy agars. Overall, the discrepancies in susceptibility results of Mueller-Hinton agar and each of Nutrient and Tryptone Soy agars were 9.9% (40/450) and 17.2% (77/450) respectively. Discrepancies in susceptibility results between Mueller-Hinton and Nutrient agars for the various drugs ranged from 3.3 to 26.7% for
Figure 2
Discussion
Drug resistance has become a very important public health issue due to the serious threat of multiply resistant pathogens. The escalation in resistance of bacterial pathogens has also made drug susceptibility testing highly crucial. In this study, the two bacterial pathogens investigated,
In this study, high levels of resistance were observed for penicillin, ampicillin, cotrimoxazole, tetracycline, and chloramphenicol. These are drugs which have been reported as having high percentage resistance for a lot of microorganisms for several years, and the rate of resistance has been rising over the years not only for clinical isolates but also for the normal intestinal flora of the healthy population (10,13,14). Conversely, lower rates of resistance were observed for gentamicin, amikacin, erythromycin and cefotaxime. Relatively, these drugs have been on the Ghanaian market for a short period of time as compared to drugs like ampicillin, and therefore may not have been subjected to high use and or misuse. In addition, some of these drugs like amikacin are expensive and may be prescribed for serious infections, thus limiting their usage. Very high resistance rates were observed for
The outcome of susceptibility testing is known to be influenced by several factors, some of which include the medium used for bacterial culture, type of drug tested, and the type of organism (7,8). These factors were also observed to influence susceptibility testing results in this study. The standard medium for the Kirby Bauer method of susceptibility testing is Mueller-Hinton agar. In Ghana, because nutrient and tryptone soy agars are sometimes used as substitutes for Mueller-Hinton, this prompted us to evaluate the quantitative effect of the two media on the quality of susceptibility testing. Previous studies on the subject have compared susceptibility testing on Mueller-Hinton agar with other standard susceptibility testing media such as Oxoid sensitivity test medium and Iso-Sensitest agar (13,14). Because nutrient and tryptone soy agars are general purpose media rather than standard susceptibility testing media, there is hardly any data comparing these media with Mueller-Hinton in susceptibility testing. In this study, using nutrient and tryptone soy agars in susceptibility testing introduced a deviation from the correct results in 8.9% and 17.2% cases respectively. The high discrepancy of susceptibility results observed between Mueller-Hinton agar and each of nutrient and tryptone soy agars for majority of the drugs tested raises doubts about the reliability of the latter two media for susceptibility testing. While similarity of susceptibility results have been reported between Mueller-Hinton agar and certain media including Oxoid sensitivity test medium and Iso-Sensitest agar, high discrepancy have been reported for other media such as Wilkins-Chalgren agar (15,16). The suitability of culture media for susceptibility testing is often associated with the composition which could affect growth of the test organism or drug activity in various ways (7,17). For media of poor suitability such as nutrient and tryptone soy agars, there is usually the presence of antagnostic substances or unsuitable pH that inhibits drug activity (7).
While the high resistance patterns observed for the organisms studied underscore the need for susceptibility testing, the findings of the study discourage the use of nutrient and tryptone soy agars in the Kirby Bauer method as practiced by some laboratories in Ghana, due to the considerable error margin these media may introduce into susceptibility results.
Acknowledgements
The technical support provided by Gloria Asala and other staff of the Microbiology Department of University of Ghana Medical School staff is gratefully acknowledged.