Goodpasture's Syndrome: A Case Report And Review Of Literature
P Mazidi, S Bajestani, M Khan, T Khair, L Laos
Keywords
anti-glomerular- basement-membrane antibodies anti-gbm, glomerular basement membrane, goodpasture's syndrome, hemoptysis, plasmaphresis, pulmonary hemorrhage
Citation
P Mazidi, S Bajestani, M Khan, T Khair, L Laos. Goodpasture's Syndrome: A Case Report And Review Of Literature. The Internet Journal of Internal Medicine. 2007 Volume 7 Number 2.
Abstract
Goodpasture syndrome is the clinical entity of acute glomerulonephritis, pulmonary alveolar hemorrhage and circulating anti-GBM antibodies. Goodpasture disease is often used to describe glomerulonephritis and the presence of circulating anti-GBM antibodies, without alveolar hemorrhage. We report a case of Goodpasture's syndrome in a 19 years old African American male who presented with pulmonary symptoms, with no renal manifestations.
Introduction
Goodpasture's syndrome is manifested by rapidly progressive glomerulonephritis and intraalveolar hemorrhage in association with the presence of anti-glomerular basement membrane (anti-GBM) antibodies. It is a rare but severe immunological disease. The diagnosis can be confirmed by the presence of circulating anti-GBM antibodies and or deposition of antibodies on the glomerular basement membrane that is usually revealed by immunofluorescence (IF) staining of the renal biopsy specimen. Here we present a case of Goodpasture's syndrome in a 19 year old man who presented with recent onset of shortness of breath.
Case
A healthy 19 year old African American man presented with shortness of breath for two weeks duration associated with subjective fever, chills and non productive cough. He was recently seen by his primary care provider with the above symptoms and was prescribed a two weeks course of Azithromycin with no clinical improvement.
On arrival to Emergency Department, he was found in mild respiratory distress with respiratory rate of 30 per minute. He was alert and able to answer all questions in full sentences. Rest of the vitals included temperature of 101.8 F (38.8 C), blood pressure of 120/60 mm Hg, heart rate of 100 beats per minutes, and pulse oximetery of 93%-98% on 40% oxygen. Head and neck examination were unremarkable. Chest auscultation revealed good air entry bilaterally with occasional bibasilar crackles and few scattered wheeze. Cardiovascular, abdominal, neurological examinations were within normal limits.
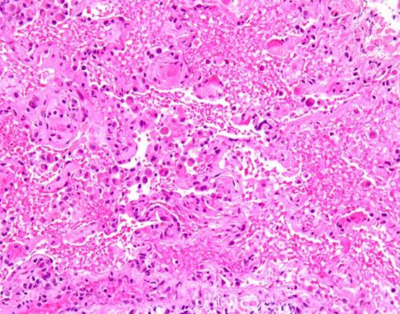
Laboratory work up was remarkable for leukocytosis, otherwise was non-contributory. Arterial blood gas was remarkable for hypoxia. (PaO2-71 mm of Hg). A chest x ray and chest CT were performed. (See figure 1 and 2). The patient's condition steadily deteriorated and was eventually intubated and ventilated requiring high peak end-expiratory pressure (PEEP). All cultures were negative. HIV antibody was negative. Patient was started on broad spectrum antibiotics with no clinical improvement. On day 3, bronchoscopy was performed due to lack of clinical improvement. Bronchoalveolar lavage was negative for PCP, AFB, fungal, HSV, Blastomycosis and Histoplasmosis. Vascuilitic and autoimmune screening was found to be negative on initial laboratory investigation. Lung tissue biopsy was performed via video-assisted thoracoscopic approach (VATS) which showed acute lung injury characterized by interstitial and intra-alveolar organization, intra-alveolar fibrin deposition, squamous metaplasia of bronchiolar epithelium and rare fibrin thrombi. These changes were associated with focal alveolar hemorrhage and capillaritis. (See figure 3). Anti-GBM is found to be 70 units (normal 0-20) which are highly positive. Therapy with prednisone, cyclophosphamide and plasmapheresis was initiated and the patient showed both clinical and serological improvement. Finally he was discharged home with a slow tapering dose of prednisone and cyclophosphamide. Upon discharge the patient was schedule for a monthly follow up.
Figure 1
Figure 2
Figure 3
Discussion
Goodpasutre first described this disorder in 1919. He reported a case of pulmonary hemorrhage and glomerulonephritis as Goodpasture disease1. In 1967, the discovery of anti-GBM led to the understanding of the pathophysiology of Goodpasture syndrome1. Anti-GBM antibody mediated disease, which typically present with the syndrome of glomerulonephritis and pulmonary hemorrhage, but may present with glomerulonephritis alone1. The interesting point about the case in question is that the above mentioned patient had pulmonary manifestation of Goodpasutre disease with high titer anti-GBM antibodies and normal renal function. (Patient refused to have kidney biopsy for confirmation of kidney involvement).
Goodpasture's syndrome is infrequent, with an incidence of approximately 0.1 cases per million population. Gender distribution is reported differently in different studies, and the age at presentation can range from the first to the ninth decade. Pediatric literature indicates no predilection in either sex2. There are no good data on the incidence or prevalence of this disease. However, acute glomerulonephritis due to anti-GBM antibody disease is rare and it is estimated to occur in less than one case per million4. Lung involvement is even rarer4. Younger patients (<30 years) are more likely to present with the full constellation of Goodpasture syndrome (eg, with pulmonary hemorrhage), and older patients (>50 years) with isolated glomerulonephritis[5,6]. There appears to be a slight male predominance in the younger age group and a female predominance in the older age group. Substantial variations exist in the clinical manifestation. 60%-80% have clinically apparent manifestation of pulmonary and renal disease, 20%-40% have renal manifestation alone, and fewer than 10% have disease that is limited to lung as in the above mentioned case1. It is worth mentioning that the above mentioned patient refused to have a renal biopsy and he may be able to have an underlying renal involvement with normal renal studies. However, renal involvement without abnormal renal parameters is very unlikely.
This is an autoimmune disorder. The auto-antibodies mediate the tissue injury by binding to their reactive epitopes in the basement membrances1. The principal component of the basement membrane is type IV collagen, which acts as a support structure and is composed of building blocks that are linked end to end1. The building blocks are composed of 3 alpha subunits of collagen, which form a triple helix. Type IV collagen can be expressed as 6 different chains, alpha 1 to alpha 6. The alpha chain itself has 3 structural domains, as follows: 1) 7-S domain at the amino terminal, 2) a triple helix of 3 alpha chains which ends at the carboxyl terminal, 3) a noncollagenous domain1. The classic triple helix is composed of 2 alpha1 chain and 1 alpha2 chain. The Goodpasture antigen has been localized to the carboxyl terminal of the noncaollagenous domain of the alpha3 chain of type IV collagen. The anti-GBS auto-antibodies (typically IgG but sometimes IgA or IgM) are directed against a 28 KD monomeric subunit present within the noncollagenous domain1.
Anti-GBS auto-antibodies also react with the pulmonary alveolar basement membrane and causes alveolar hemorrhage. These antibodies react with an epitope contained within the basement membranes. The preferential binding of the alveolar and glomerular basement membranes appear to be due to greater accessibility of epitopes in these tissues and greater expansion of alpha3 collagen units1. The variable presence of pulmonary disease appears to reflect a general lack of access of the circulating anti-GBM antibodies to the alveolar basement membrane1. Thus, patients with pulmonary involvement often have underlying pulmonary injury due to smoking, or less frequently, infection, cocaine inhalation, or hydrocarbon exposure. In our patient, he had a positive tobacco and marijuana smoking habits.
Lung involvement generally consisting of alveolar hemorrhage, affects approximately 60 to 70 percent of patients; these patients are considered to have Goodpasture's syndrome. In rare cases, pulmonary involvement predominates. Pulmonary manifestations include dyspnea, cough, sometimes hemoptysis, pulmonary infiltrates on chest x-ray, and an increased carbon monoxide diffusing capacity (DLCO) due to the presence of hemoglobin in the alveoli. Iron deficiency anemia, possibly due to prolonged pulmonary bleeding, may also be seen3.
Although some patients present with relatively mild or no renal insufficiency, this disorder is known to be associated with severe renal injury that if left untreated, progresses quickly to end stage renal disease requiring dialysis7. Plasmapheresis in combination with immunosuppressant therapy with cyclophosphamide and prednisone is the treatment of choice in Goodpasture syndrome[5, 6, 8]. Review of available reports suggests that 40-45% of patients will benefits by not progressing to end stage renal disease or death, when treated with immunosuppressive therapy in combination with plasmapheresis[4, 8,9]. Despite lack of definitive evidence of benefit, plasmapheresis is generally recommended for anti-GBM disease for two reasons: improved morbidity and mortality in the era of plasmaphereis compared to historic controls. Rapid removal of anti-GBM antibodies and complements from serum is seen in comparison with slower reduction in the levels seen with immunosuppressive agents alone 7.
The initial plasmapheresis is either daily or on alternate days. Usually four liter exchanges for two to three weeks. The patient should be reassessed at the end of these two to three weeks. Further plasmapheresis may be unnecessary if the patient has improved and there is a marked decline in serum anti-GBM antibody titers. If the patient still has hemoptysis or high antibody titers, plasmapheresis may be required for longer than 3 weeks [5,6,7].
Albumin is given as the replacement fluid during plasmapheresis. However, if the patient has had a recent renal biopsy or has pulmonary hemorrhage, then one to two liters of fresh frozen plasma should be given at the end of plasmaphresis to reverse the phresis induced coagulation factors depletion [10,11].
Plasmapheresis must be accompanied by corticosteroid and cyclophosphamide. Methyl prednisone (15 to 30 mg/kg to a maximum dose of 1000 mg IV over 20 minutes) daily for three days followed with daily oral prednisone (1 mg/kg daily to a maximum of 60 to 80 mg/day), which is usually tapered once remission is induced7. The initial cyclophosphamide dose is 2 mg/kg per day orally7. Spontaneous cessation of autoantibody formation can take 6 to 9 months or longer. The optimal duration of therapy is unknown. It is recommended that, after remission is induced, maintenance therapy with low dose prednisone and cyclophosphamide should be continued for six to nine months[4, 7].
The presented patient had regular follow up in the medical clinic and his anti-GBM titers were negative at 6 months. The patient was symptom free on low dose immunosuppressant throughout follow up (now up to 10 months).
Acknowlegment
Many regards to Archer Martin, who has helped with editing of this manuscript. Competing interests: none.