A Man with Uterine Leiomyoma
E Korashi, Nabil M El Tabbakh
Citation
E Korashi, Nabil M El Tabbakh. A Man with Uterine Leiomyoma. The Internet Journal of Gynecology and Obstetrics. 2001 Volume 1 Number 2.
Abstract
Introduction
In the human male fetus, testes develop by the 7th week and begin to secrete two hormones: antimullerian hormone (AMH) induces the regression of mullerian ducts, the analogue of the uterus, fallopian tubes and upper vagina, upon binding to a specific membrane receptors, whereas testosterone induces the differentiation of the wolffian ducts into the epididymes, vasa deferentia and seminal vesicles(1).
Persistent mullerian duct syndrome is a rare form of male pseudohermaphroditism, in which well-developed mullerian structures are present in an otherwise normal (46XY) phenotypic male[2 ,3]. It is ascribed to defects in the synthesis or action of anti-mullerian hormone (AMH).[4 ,5].
AMH is a member of the transforming growth factor-beta-superfamily of glycoprotein differentiation factors that includes inhibin and activin (6) . Uterine leiomyoma are the most prevalent tumor type in women of reproductive age and are the most common reason for hysterectomies (7 ,8) .
Cytogenetic and genetic studies have in recent years advanced our understanding of the etiology of these tumors.(8 ,9) A significant relationship exists between clonal cytogenetic abnormalities and myoma size, suggesting that chromosomal abnormalities associated with individual myoma enhance myoma growth.(10 ,11)
Persistent mullerian duct syndrome is rare and infrequently encountered by gynecologist and its association with a uterine leiomyoma in a normal phenotypic male is even more uncommon. A Medline search by the authors failed to uncover another reported case. We provide the clinical details of this case as well as the pathogenesis of persistent mullerian duct syndrome and uterine leiomyoma along with a review of the literature.
Case Report
A 28-year-old male presented with a slowly growing abdominal mass of three years duration. He had undergone an exploratory laparotomy ten years before for peritonitis secondary to a perforated appendicitis. There were no other accompanying urologic or systemic complaints. Pubic hair had been noted at 12 years of age and the patient admitted having erections although he denied sexual activity. Physical examination revealed a healthy male
Abdominal examination revealed a firm, central pelvi-abdominal swelling reaching well above the umbilicus with a good range of side-to-side mobility. Transcrotal ultrasonography was 3.8, 2.7, 2.2 cm (volume 22.5 cm3 ) on the right and 3.7, 2.8, 2.4 cm (volume 24.8 cm3 ) on the left. Both gonads were noted to have a normal echogenicity. Ultrasonography of the abdomen and pelvis revealed the presence of large pelvi-abdominal swelling with complex echotexture. Laboratory studies, including complete blood count, urinalysis, fasting & post prandial blood sugar, blood urea nitrogen, serum electrolytes, liver function tests, ECG, and chest film were normal.
Other laboratory investigations which included serum alpha-feto protein and beta-human chorionic gonadotropin, prolactin, thyroid function studies and testosterone were normal for a male, similarly estradiol, dihydroepiandrostrone, alpha-4-dione levels for aldosterone, leutinizing hormone and follicle stimulating hormone were of normal values. Intravenous pyelography demonstrated a normal kidneys, ureters and urinary bladder.
Cystoscopic examination revealed a normal male urethra, bladder neck and bladder. A postoperative study of semen showed moderate oligospermia. Chromosomal analysis revealed an XY pattern.
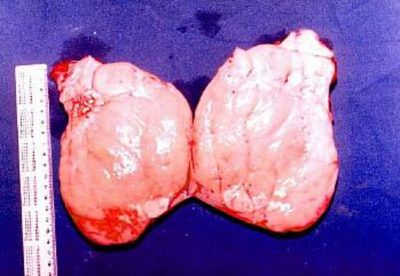
At laparotomy, a mobile large mass could be easily delivered into the laparotomy incision. Hemorrhagic adhesions bound the mass to the recto-vesicle pouch at the postero-superior aspect (uterus) from which two tubular structures (fallopian tubes) emerge from the superior aspect to stretch along the postero-lateral aspect of the large mass on each side. Each tubular structure ended in fimbriae-like processes (figs 1,2&3). The mass, together with the inseparable swelling, could be easily removed after lysis of adhesions. To avoid damage to the vas deferens, which were close to the mullerian structures and could not be separated from the vaginal wall, a small segment of the upper third of the vagina was retained. The cut section of the large mass showed a homogenous whorled pattern (Figure 4) and ,this was sent for histopathological evaluation.
Microscopic examination of the resected specimen showed that the mass was a leiomyoma (subserous) arising from the uterine wall. Dissection of the uterus revealed absent endometrial lining and the uterine corpus attached to a cervix with absent endocervical tissues. The cervix is attached to a tissue similar to the upper vaginal epithelium.
Discussion
Anti Mullerian hormone is the fetal Sertoli cell glycoprotein hormone that causes regression of the mullerian ducts in males during sexual differentiation[12,13]. The absence of AMH during female development results in the mullerian duct giving rise to the fallopian tubes, uterus, and upper third of the vagina[12]. Serum AMH values remain high (10-70 ng/ml) in human males for several years after birth and then decline to low duct levels (1-5 ng/ml) by puberty[5,14]. Several essays are currently available for the measurement of AMH in serum or plasma[14,15]. In humans, as in other species, the expression of AMH is age and gender specific from infancy to adulthood[16]. Development of the male reproductive tract depends on a multiplicity of hormones, factors and events that must interact in an appropriate time and sequence[17].
Sertoli cells of the nascent “Testis cord's” begin secreting AMH as early as the 7th to 8th fetal week, with regression of the embryonic mullerian duct completed by the 9th to 10th week of gestation[18]. In addition, there appears to be a carefully regulated ‘window' of mullerian duct responsiveness to endogenous AMH, before or after, in which AMH exposure fails to induce full regression[19].
The cloning of the gene responsible for testicular determination SRY, of the anti-Mullerian hormone and anti-Mullerian hormone receptor genes, of the several steroidogenic enzymes genes, of the 5 alpha-reductase type 2 gene and of the androgen receptor gene, has permitted researchers to elucidate the molecular defects causing abnormal sexual differentiation[20,21].
The persistent mullerian duct syndrome, characterized by the lack of regression of mullerian derivatives, uterus and tubes in otherwise normally masculinized males, is a genetically transmitted familial autosomal recessive disorder implicating either anti-mullerian hormone (AMH),or its type II receptor, a serine threonine kinase homologous to the receptor of the other members of the transforming growth factor-beta superfamily[22].
On the basis of the results of studies of human embryos and fetuses, Hoang-Ngoc, et al.,[23] believed that the myometrium is derived from the primitive mesenchyme, while the endometrium is derived from the coelomic mesothelium. The myometrium is the site of non-specific tumors common to all soft tissues (of other organs): leiomyoma, lipoleiomyoma, leiomyoblastoma, leiomyosarcoma, vascular tumors[23]. Smooth muscle tumor of uterine origin encompass a broad family of neoplasms.The leiomyoma,by far the most common of all the neoplasms,generally is hormone sensitive,with rates of growth semiquantitatively related to estrogen and progesterone receptor levels. (24)
Many researchers and clinicians have recently directed their attentions to understanding the etiology of these benign tumors, the conditions which cause their enlargement, and appropriate therapies.(25)
Estrogen receptor concentration was founded to be higher in endometrium than in leiomyomas, lowest in normal myometrium and progesterone receptors concentration in endometrium and leiomyoma were similar.(26).Estrogen and progesterone receptors expression in leiomyoma may require other extracellular factors.(27)
Estrogen and progesterone receptors are significantly more numerous in submucous than in subserosal myomas. These findings indicate that leiomyoma have a different etiology.(28) Cytogenetic and genetic studies have in recent years, advanced our understanding of the etiology of these tumors. Cytogenetic aberrations involving chromosomes 6,7,12,and 14 have been shown to constitute the major chromosomal abnormalities seen in leiomyomata. Loss of genetic material from the long arm of chromosome 7 (del 7) is critical for tumor development.(29,30)
Uterine leiomyomas are monoclonal tumors, however ,the factors involved in their initiation and growth remain poorly understood. The neoplastic transformation of myometrium to leiomyoma likely involves somatic mutations of normal myometrium and the complex interactions of sex steroids and local growth factors.(24)
Leiomyomas are rather common occurrence in the normal uterus[20]. We found no case reports of leiomyoma arising from a uterus in a male as in the case reported here. Due to the extreme rarity of leiomyoma in a male with pseudohermaphroditism, it should be considered low on the list of differential diagnosis of pelvic abdominal masses in males with mullerian inhibiting syndrome.
Correspondence to
Dr. Nabil El-Tabbakh Hadi Hospital PO Box 44630 Hawally 32061 Kuwait Tel: 965 532 7849 fax: 965 531 4717 e-mail: neltabbakh@yahoo.com