Recognising And Dealing With Artifact In Myocardial Perfusion SPECT
J Wheat, G Currie
Keywords
artifact, false negative, false positive, myocardial perfusion spect
Citation
J Wheat, G Currie. Recognising And Dealing With Artifact In Myocardial Perfusion SPECT. The Internet Journal of Cardiovascular Research. 2006 Volume 4 Number 1.
Abstract
This brief communication examines potential pitfalls in myocardial perfusion SPECT and offers strategies to prevent the loss of diagnostic integrity of data sets. In particular, this paper provides an overview of potential sources of false positive and/or false negative findings in myocardial perfusion SPECT. In the first instance, the potential sources and solutions of artifact in myocardial perfusion SPECT is discussed. Gated SPECT is introduced with respect to being a potential solution for differentiating artifact from actual defect while recognition is made of the added potential sources of error gating introduces.
Review
There has been a broad spectrum of publications and research focussed on technical and physiological artifacts in myocardial perfusion imaging. While 99mTc based myocardial perfusion SPECT imaging has a sensitivity and specificity for coronary artery disease (CAD) in the order of 91% and 79% respectively, elimination of artifacts that are known sources of false positive studies will result in improved specificity (1,2). Successful clinical utilisation of myocardial perfusion single photon emission computed tomography (SPECT) requires a high degree of technical expertise to maximise image quality and minimise the incidence of equivocal, false positive and false negative studies. There are several common imaging artifacts that can be introduced at the time of acquisition and / or during the reconstruction process.
One of the most important sources of error to consider in myocardial perfusion SPECT is patient motion (3). Patient motion is a common cause of degradation of SPECT myocardial perfusion studies because SPECT requires that the object of interest remains constant for the duration of the acquisition (4,5). Patient motion artifacts generally result in false positive studies for ischaemia with artifacts usually present in the anterior and inferior walls. Visually detectable patient motion has been reported in 36% of clinical studies in one study (3) and 43% in another (6). These figures are substantially greater than the 25% reported by Botvinick et al. (4) and the 26% reported by Prigent et al. (7) and is most likely the result of an absence of interventions to prevent or minimise patient motion in departments of data origin. It is important to recognise that visual detection of patient motion does not necessarily translate to the introduction of an artifact, however, an image artifact resulting from motion that has been misinterpreted as a true perfusion abnormality is estimated to occur in as many as 7% to 15% of patient studies (3,8).
A major source of error in SPECT reconstruction is data filtering (9,10). Filters that are too smooth may result in false negative studies and filters that are too coarse may result in false positive studies (11). Over filtering due to the method of summation of gated files in gated SPECT has also been reported to smooth the myocardial perfusion data (9). Compared to data reconstructed as ungated files, summation of reconstructed gated files was reported to result in a decrease in defect extent by 20.4%, a decrease in defect severity by 13.6%, a decrease in left ventricular lumen by 19.2%, an increase in total heart diameter by 9.8% and an increase in wall thickness by 32.3%. This introduces potential false negative results for coronary artery disease. This potential problem due to over smoothing may be particularly problematic in detecting small or non transmural defects clinically.
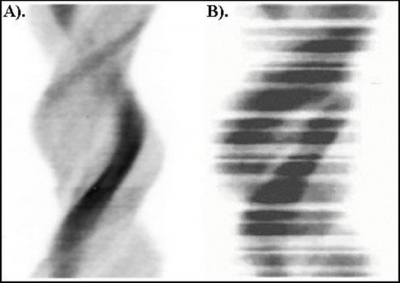
The basic principle of gated myocardial perfusion SPECT is that the functional data should not compromise the perfusion data. Bad beat rejection using a narrow window means that some data is lost that would have otherwise been included in an ungated data set. A 100% window can be used so that the functional information is not acquired at the expense of the perfusion data (i.e. accept all beats). Despite this, a number of authors recommend the use of narrower windows with 25% to 35% being typical in clinical practice (12). Not surprisingly then, Nichols et al. (13) reported that only 26% of gated myocardial perfusion SPECT patients had data sets free of gating errors while Wheat, Currie & Ramsay (6) reported 65.7% to 85.7% of gated studies to contain gating errors. Ideally, all ‘rejected' counts would be acquired in an additional 9th bin / interval so that neither the functional data nor the perfusion data is compromised. Figure 1 illustrates an example of a sinogram of the ungated SPECT data which clearly indicates the impact of beat rejection on the gated data set. The problem with this patient data set is that the gated information is unreliable and the perfusion information has been compromised.
Figure 1
There are generally three sources of soft tissue attenuation artifacts (usually fixed defects) in myocardial perfusion imaging; breast (anteroseptal, anterior and / or anterolateral walls), diaphragm and ‘cold' bowel (inferior wall) and obese patients with lateral fat pads (lateral wall). Hepatic, gall bladder and bowel activity may result in an apparent decrease in myocardial perfusion (14,15,16,17) resulting in inferior and inferolateral artifacts. These artifacts are due to the level of hepatic, gall bladder or bowel activity (compared to heart), proximity of physiological activity to heart, type and amount of smoothing before and during reconstruction, attenuation of the heart by liver and superimposition of abdominal activity over heart. These defects are generally more evident on the resting images due to the lower heart to liver count ratio compared with stress, however, pharmacological stress will also result in significantly more liver activity on the stress image. There are a number of other biological or physiological artifacts that have been reported in myocardial perfusion SPECT studies, including:
Focal increased accumulation (hot spot) in the lateral wall due to wrap around lungs,hot spot in lateral wall at “2-3 o'clock” on the short axis slice,upward creep if the acquisition is started too soon following stress,septal or anteroseptal artifact due to left bundle branch block,there are several normal variants which may mimic CAD with the most notable being apical thinning and papillary muscles (anterolateral and posterolateral defects).
Biological and physiological artifacts in myocardial perfusion SPECT, their causes and the actions to be taken to overcome them are tabulated below (Table 1).
Figure 2
Performed with a high degree of attention to detail during data acquisition, a rigorous post acquisition quality control protocol, an understanding of the potential impact of various study confounders and an awareness of interventions to circumvent such potential, myocardial perfusion SPECT can be performed with high efficacy.
Correspondence to
Dr Janelle Wheat School of Biomedical Sciences Locked Bag 588 Charles Sturt University Wagga Wagga 2678 Australia Telephone: 61 2 69332750 Facsimile: 61 2 69332866 Email: jwheat@csu.edu.au