Prevention of Hypothermia During Interventional Cardiology Procedures in Adults
K Wagner, C Smith, K Quan
Keywords
gel pad warming, hypothermia, interventional cardiology
Citation
K Wagner, C Smith, K Quan. Prevention of Hypothermia During Interventional Cardiology Procedures in Adults. The Internet Journal of Anesthesiology. 2009 Volume 24 Number 1.
Abstract
Objective:The purpose of this prospective, randomized study was to evaluate the ability of a reusable gel pad system to maintain perioperative normothermia. Methods: 95 adults undergoing elective electrophysiology surgery for arrhythmias or cardiomyopathies scheduled to last > 120 min and requiring anesthesia services were randomized into 2 groups: gel pad warming (set point = 42 oC); and controls: no active warming. The gel pad was heated prior to patient entry in the room. Control patients lay upon the unwarmed gel pad. Results. Final temperature was higher in the warmed vs control group (mean + SD 36.4 + 0.5 oC vs 35.4 + 0.8 oC, P< 0.001). No patient had evidence of pressure sores. There was no difference in fluoroscopic image quality between the two groups. The gel pad did not interfere with permanent pacemakers or defibrillators or with ablation generators.Conclusion. Gel pad warming was effective in maintaining normothermia.
Introduction
Interventional cardiology procedures are often prolonged and may involve considerable physiologic trespass in patients with limited physiologic reserve. It has been our impression that patients undergoing cardiac electrophysiology surgery (EPS) are at risk of developing hypothermia even without administration of general anesthesia, likely due to a combination of factors such as heat loss to a cold operating room, impaired thermoregulation from anesthetic sedative drugs, infusion of unwarmed IV fluids, and redistribution of heat from the core to the periphery. 1 Hypothermia is considered detrimental for the patient, and may result in shivering, increased myocardial and circulatory stress, postoperative wound infections, perioperative bleeding, prolonged hospitalization, and cardiac events such as myocardial ischemia and ventricular tachycardia. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Convective warming together with IV fluid warming are common methods for maintaining normothermia in patients undergoing major surgery with general anesthesia, and is considered routine practice. However, use of these warming devices is not generally employed during EPS because of limited surface area to apply the warming blanket and low IV fluid requirements. Other methods of maintaining normothermia such as radiant heat interfere with fluoroscopic imaging, especially since the x-ray cameras are bulky and are often moved during the surgery.
Circulating warm water gel pad mattress is an attractive method to maintain normothermia. Circulating warm water mattress exchanges heat by conduction, and can be applied to the fluoroscopy table such that there is no interference with the EPS. Moreover, encapsulating the water mattress within gel pads can enhance contact between the mattress and the patient’s back, further improving heat exchange. However, the use of gel-coated circulating warm water mattress posteriorly has been questioned due to concerns that little heat is lost from the back, and there is limited potential for heat transfer to the back. 11
The objective of this prospective, randomized study was to evaluate the ability of a gel pad warming system to maintain normothermia during EPS. The gel pad warming system was compared to routine thermal care.
Methods
Adult patients undergoing elective EPS procedures for arrhythmias (radiofrequency ablation of complex arrhythmias, pulmonary vein antrum isolation for atrial fibrillation) or cardiomyopathies (biventricular lead placements, lead extraction/revision) scheduled to last > 120 min and requiring anesthesia services were randomized into 2 groups: gel pad warming; and routine thermal care (controls- no active warming). The protocol was approved by the Institutional Review Board. Written informed consent was obtained. Exclusion criteria were age < 18 or > 85 years, malignant hyperthermia, preoperative temperature >38 o C or <35 o C, and lead infection. Patients were identified through the daily EPS schedule. A random number generating algorithm was used for group assignment. The anesthesia and nursing staff were aware of patient group. Cardiology physicians were blinded to patient assignment. Patients were enrolled over a 12 month period between Aug 2007 and Aug 2008.
In the warmed group, the full body water warmed gel pad (Gelli-Roll: 186.7 cm x 54.61 cm pad, Cincinnati Sub-Zero, Cincinnati, OH, Figure 1) was positioned on the surface of the fluoroscopic table. The pad was heated by a water warming unit with high flow rate (Blanketrol, Cincinnati Sub-Zero) prior to patient entry in the room and continued intraoperatively (set point = 42 o C). A single hospital sheet was placed on top of the gel pad upon which the patient lay supine with the arms tucked at the side. Control patients lay upon the unwarmed gel pad and sheet that was not connected to the Blanketrol unit.
In both groups, the patient wore a standard hospital gown and was covered by a single bath blanket before start of anesthesia. The gown and bath blanket were removed for placement of monitors and defibrillation pads and then reapplied until prepping and covering with sterile drapes.
Choice of anesthesia technique (deep sedation vs general anesthesia), airway management, and anesthesia drugs was at the discretion of the attending anesthesiologist and not dictated by protocol. The ambient temperature was set at 21 o C. IV fluids were not warmed.
Preoperatively, baseline sublingual temperatures were measured with an electronic thermometer (IVAC Temp Plus II thermistor, IVAC Corp., San Diego, CA). Sublingual placement and mouth closure were carried out during all measurements. Intraoperatively, nasopharyngeal or distal esophageal temperature were measured at 15 minute intervals until the end of the procedure (final) using an 18 or 9 Fr esophageal stethoscope with temperature sensor (Thermistor 400 series, Tyco Healthcare, Pleasanton, CA). Patient and procedure room temperature were displayed on a 2 channel monitor (Mon-a-therm, Model 4070). Vital signs, including oxygen saturation and end-tidal CO2, were measured preoperatively and at 3-5 minute intervals during the procedure.
Cardiology physicians were questioned about the quality of the fluoroscopic image. Any interference with monitoring, grounding, and defibrillation were noted during the surgery. At the end of the surgery, the patient’s skin was inspected by the cardiology nurse for any sign of redness, swelling, or blisters (signs of thermal injury or pressure sores). 12 The computerized hospital chart (EPIC) was reviewed for complications up to two weeks post-procedure. The primary hypothesis was that patients in the gel pad warming group would have higher temperature at the end of the procedure compared with controls. Data (reported as means + SD or number of patients and percent) were compared between groups by using t tests and repeated measures ANOVA for continuous valued variables, chi-square tests for categorical variables, and Fisher exact test when variable categories included fewer than five patients. Statistix 8.0 software was used. A P value < 0.05 was considered significant.
Results
Ninety five patients were enrolled: 48 in the gel pad group and 47 controls. Three patients were excluded in the gel pad group (lead infections). Two control patients were excluded (1 - percutaneous coronary intervention prior to EPS procedure; 1- infected leads). There were no differences between groups with respect to preoperative and intraoperative variables (Tables 1 and 2), co-morbidities, cardiac medications, anesthetic technique, airway management, anesthesia drugs, blood loss, and fluid requirements.
Abbreviations: ASA- American Society of Anesthesiology. COPD- chronic obstructive pulmonary disease. ACE- angiotensin converting enzyme. Data are means + SD or numbers of patients. Some patients had more than one indication for the procedure. There were no significant differences between groups.
Data are means + SD or number of patients (%). * P < 0.001 between groups
The majority of patients (77 -78% ) had deep IV sedation and breathed supplementary oxygen through a nasal cannula or plastic face mask. The primary agent was propofol (2130 + 1258 mg) in 84% of patients supplemented with fentanyl (171 + 127 mcg) in 96% of patients. In 21% of patients, the clinician suspected that nasopharyngeal temperature was falsely low due to high flow nasal and mask oxygen. In those situations, the site was switched to the axilla and a skin temperature probe (Mon-a-therm Thermistor YSI 400 series, Mallinckrodt, St Louis, MO) was used. The number of patients having axillary temperature measures was similar between groups.
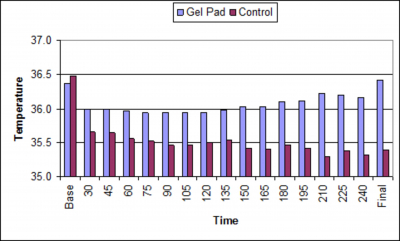
Compared with controls, patients in the warmed group had higher temperature during and at the end of the procedure, and were less likely to be hypothermic at the end of the procedure (P < 0.001, Table 2, Figure 2).
Figure 4
There were no thermal injuries or pressure sores observed or reported. There was no difference in hospital length of stay (median= 1 day, range 1-11 days), other vitals signs (blood pressure, pulse, respiration, oxygen saturation) or complications (neck or groin hematoma, pericardial effusion, hypoxemia, congestive heart failure, fractured sheath, urinary tract infection, hypoglycemia, severe pain) between groups. There was no interference with fluoroscopic image quality, physiologic monitoring, defibrillation devices or grounded signaling systems by the gel pad warming system.
Discussion
The main findings of this study are that gel pad warming of the patient’s posterior surface resulted in higher temperature compared with controls and a low incidence hypothermia. To the best of our knowledge, there are no studies of temperature management during EPS, and no studies evaluating gel pad warming during these surgeries. The findings of our study showing a 77% incidence of hypothermia in control patients is in agreement with clinical experience in other anesthetizing locations that unwarmed patients given anesthetic doses of standard agents often develop hypothermia in a cold operating room. Perioperative hypothermia is widely recognized as a contributing factor to the development of surgical site infections, increased hospital length of stay, and increased risk for adverse outcomes. 1,4,5, 10 Perioperative hypothermia may complicate the risk of the EPS, especially in patients with co-morbidities such as heart failure, ischemic heart disease, cerebrovascular disease, renal dysfunction, diabetes mellitus, and pulmonary disease. Although convective warming is a well documented method for preventing hypothermia, we have found this method difficult to apply during invasive cardiology procedures because of the requirement for exposure of the groin and upper body for access and imaging.
Gel-coated circulating water mattresses exchange heat to the patient’s back side by conduction. Conductive heat exchange is a function of the heat exchange coefficient, temperature gradient between the gel pad and the skin, and contact area. 13 There is a linear relationship between heat flux and gel pad temperature as demonstrated by Brauer et al in awake volunteers using a 56.5 x 76 cm (0.43 m 2 ) system. 12 At temperature of 41 o C, there was a net heat gain from the gel pad of 47 W per m 2 back area which resulted in a heat transfer of 18.4 W. The gel-coated water mattress had a high heat exchange coefficient of 121 W.m -2 . o C -1 because the gel coating enhanced contact between the mattress and back, reduced thermal contact resistance, and increased the efficacy of heat exchange. 12 Of note, heat exchange coefficients of convective warmers (13-35 W.m -2 . o C -1 ) are considerably lower than those of conductive warmers, which may result in lesser amounts of heat transfer depending on characteristics of the convective warming system such as nozzle temperature, air flow, temperature distribution inside the blanket, contact area, and other factors. 14 , 15 Use of the gel pad conductive warming system also serves to eliminate heat loss due to conduction, reduce the risk of pressure sores, and decrease the need for additional temperature management strategies such as convective warming, IV fluid warming, or increased ambient temperature. Gel pad heating may be applicable to other operative settings and may also alleviate cold and back pain during long procedures done in the supine position under local anesthesia. Efficiency of the gel pad may be reduced by the imposition of a blanket between the gel pad and the patient.
The study was not designed to detect a significant difference in length of hospitalization, blood loss, and infection. These outcomes have previously been addressed. 16 Core temperature measurements were not able to be done in all patients and an intermediate site (axilla) was used in 21% of patients. Axillary temperature provides an estimate of core temperature, but response time is altered by volume of tissue and fat separating peripheral compartments from perfused vasculature. Final temperature differences did, however, remain highly significant between groups when stratified for temperature monitoring site. Further, there were no differences in final temperature within each group for the different monitoring sites. No attempt was made to control the type (deep sedation vs general anesthesia) or amount of anesthetic agent in the study. For example, general anesthesia with endotracheal intubation was the preferred technique in patients with sleep apnea and in patients requiring intraoperative transesophageal echocardiography. There were no differences in anesthetic technique and drug dosages between groups. Thus, it is assumed that thermoregulatory control would be impaired in both groups to a similar degree.
In summary, full body gel pad warming resulted in higher procedural temperatures and a lower incidence of hypothermia compared with controls. Advantages of the gel pad warming system include patient warmth and no interference with fluoroscopic imaging.
Acknowledgements
The authors are grateful to anesthesia and cardiology personnel at MetroHealth Medical Center for their participation, Saied Amini PhD, MBA, JD for statistical consultation, and Doug Knierman CRNA and Maralyn Glendenning RN, CRNFA for assistance with enrolling patients. Cincinnati Sub-Zero provided an educational grant of $4500 to the MetroHealth Medical Center Dept of Anesthesia.