A New Personal Percutaneous Tracheostomy Approach: A Preliminary Animal Study
E Manno, L Bertolaccini, A Berra, S Burchielli
Keywords
animal model, new technique, percutaneous tracheostomy
Citation
E Manno, L Bertolaccini, A Berra, S Burchielli. A New Personal Percutaneous Tracheostomy Approach: A Preliminary Animal Study. The Internet Journal of Anesthesiology. 2007 Volume 17 Number 1.
Abstract
Disclosure
Emilpaolo Manno has a financial interest in Mini Invasive Tracheostomy kit.
Luca Bertolaccini, Alessandro Berra, and Silvia Burchielli have no financial interest in Mini Invasive Tracheostomy kit. The study was performed with the financial support of X-med (Modena, Italy).
Introduction
Percutaneous tracheostomy has now been widely adopted for the long-term airway management of critically ill patients and represents one of the most frequently performed surgical procedure in Intensive Care Unit (ICU). Currently, five different types of percutaneous techniques are used [1, 2, 3, 4]; and the first two proposed, the original Ciaglia method [1] and the Griggs forceps [2] are still widely used despite new approaches. This fact can mean, only, that ICU physicians are not completely satisfied with actually used techniques and the need for an improved dilatative technique is therefore obvious [5, 6, 7]. Actually, the percutaneous tracheostomies techniques do not use the available technologies as it has been done for example in cardiology, radiology, or endoscopy [8].
The aim of the study was to evaluate a tracheotomy device; witch comprises a cannula that passes from a contracted status, inside a peel-away introducer, to an expanded status to dilatate neck tissues performing a tracheostomy.
Methods And Materials
The experiments has been conducted in accordance with national (Law n° 116/92, n° 8/July 94) and international Laws and policies (European Economic Community, Council Directive 86/609, December 1987). The animals were maintained in a pigpen for 1 to 3 days with free access to water and food pellets before enrolment. They were fasting the night before the procedure.
All the procedures were performed by the authors, skilled in dilatative tracheostomy.
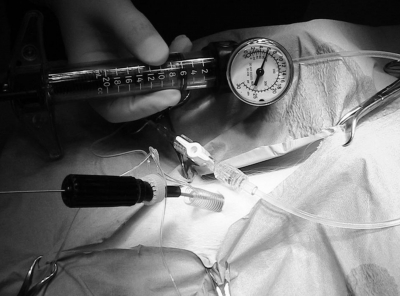
As a matter of fact, a color-coded filling channel was filled with saline under pressure (from 6 to 12 atmosphere (atm), [corresponding to 6079.5 – 12159 hectopascal (hPa)]) using QL ® Inflation Device (Atrion Medical Products Inc., Arab, AL, U.S.A.). As it is preformed and made of not expansible material, the cylinder dilates the pig's tissues circularly and symmetrically, and assumes an expanded configuration typical of a tracheostomic cannula. After this, a second color-coded filling channel was filled with saline under pressure (up to 4 atm [4053 hPa]) and a preformed balloon (Figure 2) reached a predetermined shape.
The aim of balloon was to obtain an optimal dilatation of tracheal rings. 30 seconds later, the balloon was deflated and was removed together with the guide-wire. An inner cannula with an obturator was inserted, the obturator was removed, and air was fed into the airtight cuff of the cannula by means of a second color-coded inflation line (Figure 3).
The tracheostomy cannula was secured to the skin. The orotracheal tube was removed and the pig was ventilated trough the tracheostomy. Anesthesia drugs were stopped and pig awaked and begun spontaneous breathing. The animals were sacrificed one hour after the procedure, with intravenous injection of Propofol (Diprivan ®, Astrazeneca S.p.A., Basiglio (MI), Italy), 10 mg/kg and Potassium Chlorite, 20 mEq.
The length of procedure was evaluated in minutes, as the time from insertion of the guide-wire into trachea to expansion of shape memory cannula (time a), as the time from insertion of the inner cannula to pig ventilation (time b), and as total time (time c). We used the classification by Frova and Quintel (4) to evaluate difficulties in performing the procedure: (I) easy; (II) some difficulties, but possible; and (III) impossible. Bleeding, during the procedure, was classified as absent/minimal (≤ 10mL), medium (11 – 50 mL), and serious (≥50 mL). We evaluated also posterior tracheal wall damage, kinking of guide-wire, tracheal rings fracture, and para-tracheal insertion.
Results
The results are shown in Table II.
Mean time (± standard deviation [SD]) from insertion of the guide-wire into trachea to expansion of shape memory cannula (time a) was 6.20 ± 0.03 minutes. Mean time (± SD) from insertion of the inner cannula to pig ventilation (time b) was 2.04 ± 0.01 minutes. In addition, mean (± SD) total time (time c) was 8.24 ± 0.04 minutes. There were some difficulties in performing two procedures. Bleeding was absent in 4 (66.7%) pigs, and minimal (≤10 mL) in 2 (33.3%) pigs. There were no cases of posterior tracheal wall damage (Figure 4), no cases of tracheal rings fracture, and no cases of para-tracheal insertion of the device. In 2 (33.3%) cases, we observed kinking of the guide-wire.
Discussion
In elective tracheotomy, fast procedure is not essential. Even if it is not possible to compare the procedure described in our paper with other human studies, too long a procedure could be a problem for ICU physicians. The mean time from guide-wire insertion to ventilation of the pig in our study was 8.24 ± 0.04 minutes. This time can be considered as satisfactory for an elective tracheotomy.
Literature described a learning curve for percutaneous tracheostomies, resulting in a decreased rate of complications and in a shorter procedure with appropriate experience [9]. The technique described in our paper has a simple learning curve. The range in length of procedure has oscillated between 9.10 minutes to 7.05 minutes.
Tracheal endoscopic examination is usually advocated for routine percutaneous tracheostomy, but we did not perform it in our study; nevertheless, we had only negligible complications, as minimal bleeding and kinking of the guide-wire. After sacrifice of pigs, we saw neither tracheal rings fracture, nor posterior wall lesions. These experimental evidences suggest that the progressive radial dilatation is protective against two complications that can lead to late tracheal stenosis [10, 11].
In conclusion, The Mini Invasive Tracheostomy kit is easy to perform and safe. The next experimental step for our device will be a perspective human study that will achieve the evaluation of the long term complications as excessive granulation tissue and the subsequent tracheal stenosis.
Correspondence to
Emilpaolo Manno, MD PhD Emergency Department – Maria Vittoria Hospital 72, Cibrario Street – 10149, Turin (Italy) Phone and fax: +39-011-4393412 E-mail address: e.manno@asl3.to.it