Ureteroarterial Fistulas: Current Diagnostic and Treatment Considerations
D Point, J Hakim, V Chander, A Luchey, S Zaslau
Keywords
hemorrhage, trauma, ureteroarterial fistula
Citation
D Point, J Hakim, V Chander, A Luchey, S Zaslau. Ureteroarterial Fistulas: Current Diagnostic and Treatment Considerations. The Internet Journal of Urology. 2010 Volume 8 Number 1.
Abstract
Ureteroarterial fistulas (UAF), a rare cause of hematuria [1,2] with a mortality rate reported as high as14-23% [2,3] were first reported in 1908 [4]. Although there has been an increase in incidence, reports in the literature consist only of case review and a single institution case series [2]. The identified predisposing factors for UAF formation are chronic ureteral catheterization, previous pelvic surgery, radiotherapy and vascular disease with previous vascular surgery or grafting [2]. Additional awareness of this complication may possibly aid in identification of high risk individuals and preventive measures. We present a review of UAF focusing on identifying patients at risk for fistula development, presentation, diagnostic imaging/procedures, and treatment modalities.
Introduction
Ureteroarterial fistulas (UAF), a rare cause of hematuria [1,2] with a mortality rate reported as high as14-23% [2,3] were first reported in 1908 [4]. Although there has been an increase in incidence, reports in the literature consist only of case review and a single institution case series [2]. The identified predisposing factors for UAF formation are chronic ureteral catheterization, previous pelvic surgery, radiotherapy and vascular disease with previous vascular surgery or grafting [2]. Additional awareness of this complication may possibly aid in identification of high risk individuals and preventive measures. We present a review of UAF focusing on identifying patients at risk for fistula development, presentation, diagnostic imaging/procedures, and treatment modalities.
Presentation
UAF can potentially result in rapid lethal hemorrhage; therefore prompt diagnosis and intervention is important in the management of this condition. Patients can present with either microscopic hematuria, intermittent gross hematuria or with life threatening exsanguinating hemorrhage [5]. Prior to 1980, the mortality rate was reported to be as high as 69%; however, with the addition of new diagnostic and therapeutic interventions, coupled to an increase in awareness, decreased mortality rates as low as 14-23% have been reported [2,3,6]. This was highlighted in a review of 139 cases where a mortality rate of 13% was cited, despite identifying only 22% of fistulas prior to treatment [6]. These statistics highlight the importance of prompt diagnosis and need for potentially invasive procedures to determine and treat the fistula. The most common presentation of UAF is intermittent or continuous gross hematuria. In rare presentations, patients demonstrate symptoms of coexisting hemorrhagic shock due to blood loss [2,3,7]. In about half of the cases coexisting hydronephrosis, due to ureteral occlusion by clots, patients have reported flank and/or groin pain that typicallylateralizes to the side of the fistula [2,7]. Gross hematuria can result in obstruction due to clot formation, resulting in lower urinary tract obstructive symptoms such as urinary hesitancy and increased urinary frequency. Several risk factors have been recognized and are thought to have a direct relationship to fistula formation. The following section discusses thesr risks which should alert clinicians to the possibility of UAF.
Risk Factors
There has been a dramatic increase in the incidence of UAF for several reasons including: the introduction of the ureteral stents, aggressive surgical resection and radiotherapy in the treatment of pelvic malignancy [1]. A review of 37 cases identified several predisposing risk factors for fistula formation: previous genitourinary or pelvic surgery (68%); chronic ureteral stent placement (65%); radiation therapy (46%); previous vascular surgery (19%); and underlying vascular pathology, such as atherosclerotic aneurysms (19%) [5]. Similiar studies have since confirmed these findings, with history of prior pelvic surgery, pelvic radiation and indwelling ureteral stents identified as the most highly associated conditions with UAF [2,5,6,8]. These factors appear to be additive and there is an association with carcinomas of the cervix, bladder, endometrium, rectum and vulva most likely due to the combined use of surgery, radiation, and ureteral stenting in disease management.
Other less commonly identified conditions reported with UAF are abdominal aortic aneurysms, ureteral lithiasis, prior balloon dilation of ureteral strictures, appendectomy, pelvic abscesses, external penetrating trauma, diverting urinary conduits, pelvic vessel aneurysms and pyelonephritis during pregnancy [1,5,6,9]. Idiopathic ureteroiliac fistula are extremely rare and are reported to constitute less than 15% of fistulas, with aorto-iliac aneurysm being the dominant finding, and rarely arteriovenous malformation or aberrant vasculature observed during surgical exploration [1].
Pathophysiology
The first reported case occurred following open bilateral ureterolithotomies [4]. The fistulas were a result of compression due to placement of external drainage tubes and the complication was identified after their removal prompted gross hematuria and external hemorhage. The current increase in the incidence of this rare condition appears to be associated with the expanded use of indwelling ureteral stensts, suggesting a related mechanism for fistula formation. UAF frequently occur where the ureter crosses over the artery, with the ipsilateral common iliac artery being the most common followed by the external and internal iliac arteries [2]. Most UAF are thought to arise as a result of fibrosis following surgery or radiation which immobilizes the ureter to the artery at the point of intersection. Atheroscerotic aneurysms may generate perivascular inflammation and contribute or produce local fibrosis. Following fibrotic attachment of the ureter to the artery; the presence of an indwelling ureteral catheter serves as a pressure counter point. The constant pulsation of the iliac artery is then transmitted to the already damaged ureter and artery. This stress generates pressure necrosis with eventual fistula formation. Factors that promote tension and damage to the lining of these structures (hydroureteronephrosis, infection, interruption of blood supply and innervations second to surgery, hypotension, and radiation) generate further compromise [7]. The presence of these conditions can weaken wall strength of both structures, and precipitate or worsen fistula hemorrhage during ureteral stent exchange [7]. As a result Van den Bergh et al. recommend the use of smaller softer stents in patients requiring chronic ureteral catheterization with a coexisting history of pelvic radiation or surgery to prevent this rare but devastating complication [10]. The fact that a 7 French stent provides the same flow rate as a 12 French further supports the argument, however, UAF have been reported despite use of smaller stents. Therefore a smaller French stents do not exclude possible fistula formation [9,11].
Diagnosis
Demonstrating a fistula can be very challenging, and a high index of suspension is needed due to the lack of sensitive diagnostic tools. Clotting and valvular action of ureteral stents can result in intermittent hemorrhage making visualization difficult. CT imaging was reported in one study to have a sensitivity of only 50% due to the small size of many fistulas, and the interference caused by the high contrast interference of indwelling ureteral catheters [2]. The presence of a pseudoaneurysm can indicate the existence and location of a fistula, and identify additional pathology such as fluid collection, pelvic masses and hydronephrosis (Figure 1) [2,12,13]. Unfortunately these findings are nonspecific, and are often suggestive in retrospect after diagnosis has been confirmed through other studies. Similarly retrograde pyelogram was reported to have a sensitivity of 45-60% [5]. However, these studies can demonstrate possible casting off the collecting system suggesting thrombi and demonstrate possible obstruction due to stricture at the site of the fistula (Figure 1). While not definitive, these findings indicate the need for more invasive diagnostic procedures.
Figure 1
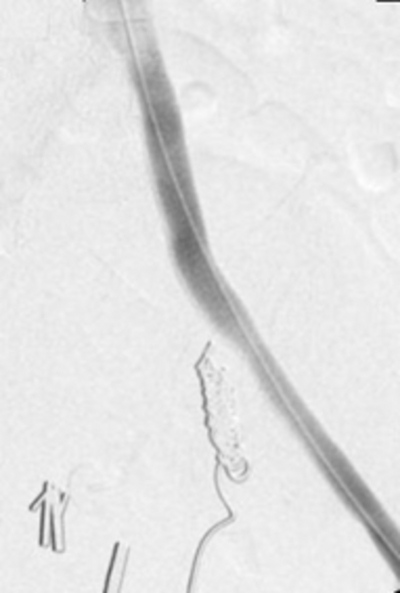
Due to the intermittent nature of fistulas and small amount of contrast extravasation into the ureter relative to the artery, angiography has been reported to have a low sensitivity ranging from 23 to 41% [2,6,9]. Traditional angiography can yield nonspecific evidence of a fistula or aid in determining location (Figure 2). Provocative techniques such as stent or arterial catheter manipulation, and direct fistula catheterization have been used to induce or increase bleeding (Figure 3). These techniques allow for radiologic demonstration of the fistula with an increased diagnostic yield as high as 63 to 100%.[14,] Due to the risk of complications including hemodynamic compromise, provocative maneuvers should be undertaken with the support of a multidisciplinary team to manage complications and allow for emergent treatment [6,9]. The use of intravascular ultrasound (IVUS) to demonstrate a pulsatile flow pattern in the ureter, may aid in diagnosis during acute hemorrhage despite negative angiography, however, no reports in the literature have determined the sensitivity of this tool.
Figure 2
Figure 3
Ureteroscopy has been used to visualize fistulas, but high pressure irrigation is often needed to allow visibility and there is the possibility of inducing or exacerbating hemorrhage. Additionally direct visualization of a lesion can identify ureteral perforation, without allowing for a definitive diagnosis of UAF. A retrospective series found that ureteroscopy with provocative angiography was diagnostic in 63% [8]. Cystoscopy might reveal ureteral blood flow that may or may not be pulsatile, however the sensitivity remains low. One group reported improved detection by employing a method they termed “antegrade nephrostogram using retrograde access.” By placing a ureteral catheter and injecting contrast while gradually withdrawing, they reported a localized reversal of the pressure gradient at the site of the fistula that demonstrated extravasation of contrast into the aorta during diastole [3]. In a study of 11 patients Van den Bergh et al. found that 36% had negative ureteral contrast and non-provocative angiographic series, and diagnosis required exploratory laparotomy [6]. Currently the only published diagnostic algorithm for UAF management has been proposed by Krambeck et al. based on 8 patient single institution case series [2].
Treatment
Advances in therapeutic techniques have reduced the mortality rate for UAF in recent years [9]. Various treatment options for UAF exist addressing both the arterial and ureteral components of the fistula. The immediate goal of therapy is establishment of hemodynamic control. Patients with a picture of evolving shock may require emergent ureteral or arterial embolization, endovascular stenting and/or open exploration with delayed reconstruction until stabilization is achieved. While open reconstruction was the treatment of choice and has demonstrated excellent results in the past, the comorbities, need for emergent vascular control, history of prior radiation, and pelvic surgeries associated with fistula formation have made this approach less appealing in recent years due to adhesions and other complications including wider incisions needed to establish vascular control [6,12]. When open surgery is selected, primary repair of the both the artery and ureter are allowed, with omental wrapping demonstrating a benefit in preventing reoccurrence [1].
Open surgery is indicated when endovascular approaches fail to identify the location of the fistula, little renal function remains in the affected kidney or emergent vascular control is needed to stop blood loss. Due to the risk of severe hemorrhage, vascular management is the deciding factor when selecting treatment, and is dependent on the presence of infection or abcess, aneurysm or occlusive arterial disease and the availability of collateral blood flow to the ipsilateral lower extremity [5]. The artery can be managed by primary open repair, open ligation, or embolization followed by extra-anatomic reconstruction to preserve lower extremity profusion, or endovascular stenting [3]. Similarly, the ureteral component of the fistula can be managed with nephroureterectomy with primary repair of the arterial component, nephrostomy tube placement with or without simultaneous stenting, ureteroureterostomy, transureteroureterostomy, cutaneous ureterostomy, or percutaneous nephrostomy with ureteral ligation [5].
Currently endovascular stenting has become the treatment modality of choice due to its minimally invasive approach, rapid control of bleeding, and rapid patient recovery times. Additionally, it can be rapidly initiated following provocative angiography [2,9,13,16]. Previously balloon-expandable autologous vein covered grafts were the treatment of choice, however they have largely been replaced by self-expandable synthetic resin stents with good results (Figure 6) [2,6,13]. However, the long term graft patency and infection rates of these grafts have yet to be evaluated. Without active bleeding to identify fistula location common iliac arterial embolization can be difficult. Endovascular stenting has the several advantages over embolization; arterial supply to the lower extremity is preserved excluding the need for a secondary arterial bypass graft along with its associated complications and visualization of the fistula is not required [16]. The internal iliac artery is often embolized in the case of a fistula located at in the common iliac in order to successfully ensure graft placement and vascular control (Figure 4). Figures 5 and 6 show the successful tamponade and outcome of the UAF.
Figure 4
Figure 5
Figure 6
In regards to the ureter, Kim et al. recommend that it be managed by leaving an indwelling ureteral catheter in place, citing its aid in several management options. In the case of open reconstruction, placement can aid in early identification of structures. Additionally it can serve as a landmark in the deployment of endovascular stenting and nephrostomy tube placement while serving to minimize vascular stent infection [3]. The addition of new diagnostic and therapeutic approaches has significantly reduced the need for exploratory laparotomy, ipsilateral nephrectomy, renal artery embolization, and ureteral ligation in the management of UAF [7]. However, more definitive long term studies are necessary to evaluate the long term outcomes of different treatment modalities.
Conclusion
Ureteroarterial fistulae are a rare cause of both intermittent and continuous hematuria. Due to the lack of definitive diagnostic studies, and the high rate of mortality associated with delays in diagnosis, clinicians must maintain a high index of suspicion when addressing patients with gross hematuria and other risk factors that are concerning for UAF. Identification of predisposing factors can lead to prompt diagnosis or suggest the need for emergent laparotomy in the presence of negative diagnostic studies. Additional treatment procedures, including leaving ureteral catheters in place following hemorrhage can aid in management. Traditional imaging studies may lend support to the diagnosis, warranting the use of potentially more invasive procedures to yield the diagnosis. Endovascular stenting has become the treatment of choice in recent years with satisfactory results reported in the literature.
Key Points: Ureteroarterial Fistula
Patients with UAF usually have one or more of the following risk factors:
1. Indwelling ureteral stents
2. history of prior pelvic surgery
3. radiotherapy.,
incidence is increasing, and no definitive diagnostic modalities are currently available, requiring a high degree of suspicion
ients who present with hematuria and have a history of risk factors, require more aggressive diagnosistic and theraputic treatment due to the high mortality associated with the condition
rent strategies in management are based on establishing vascular control initially with an endovascular approach and angioplasty or stent placement to tamponade/occlude bleeding, with progression to open procedures if stability cannot be obtained.