The sperm count has been decreasing steadily for many years in Western industrialised countries: Is there an endocrine basis for this decrease?
S Dindyal
Citation
S Dindyal. The sperm count has been decreasing steadily for many years in Western industrialised countries: Is there an endocrine basis for this decrease?. The Internet Journal of Urology. 2003 Volume 2 Number 1.
Abstract
There have been a number of studies over the past 15-20 years (1, 2, 3, 4, 5, 6 and 10), which suggest that sperm counts in man are on the decline. Since these changes are recent and appear to have occurred internationally, it has been presumed that they reflect adverse effects of environmental or lifestyle factors on the male rather than, for example, genetic changes in susceptibility. If the decrease in sperm counts were to continue at the rate that it is then in a few years we will witness widespread male infertility. To date it remains unknown why this is happening and the available preventative measures, which can be taken to avoid a continuation of this trend, are not common knowledge.
Introduction
For a healthy male, typical seminal fluid analysis values should be (13):
However, according to the ever-increasing literature on sperm counts, these “normal” values are steadily decreasing and only a minute proportion of males will have semen values that satisfy these ideal figures in today's Western industrialised countries. Not only are sperm counts decreasing, but also are the average sperm volumes which contain a greater proportion of deformed spermatozoa that have reduced motility's.
Professor Niels Skakkeback, a Danish scientist, first alerted the world to the possibility of a substantial fall in male fertility levels in 1992. He did this by showing that sperm counts in healthy men appeared to have dropped by more than half in 50 years (1, 2, 3, 4, 5, 6 and 10). Professor Skakkebaek's work attracted worldwide publicity at first – and then ridicule. He and his team in the Department of Growth and Development at Copenhagen University had reviewed 61 international studies involving 14,947 men between 1938 and 1992 (4). They found that the average sperm count had fallen from 113 million per millilitre in 1940 to 66 million in 1990. In addition, the definition of a “normal” sperm count fell from 60 million per millilitre to 20 million in the same period (1). Critics who reanalysed the Danish data pointed out a fundamental flaw in the calculations which, they said, ruled out any significant decline.
Subsequent studies have confirmed and strengthened Skakkebaek's findings. A survey of 1,350 sperm donors in Paris found a decline in sperm counts by around 2% each year over the past 23 years, with younger men having the poorest-quality semen (1, 5 and 6). In another study at the University of Helsinki led by Jarkko Farjarinen, testicular tissue was examined at post-mortem from 528 middle-aged Finnish men who died suddenly in either 1981 or 1991 (11). Among the men who died in 1981, 56.4% had normal, healthy sperm production. By 1991, however, this figure had dropped dramatically to 26.9%. The average weight of the men's testes decreased over the decade, while the proportion of useless fibrous testicular tissue increased. Adamopoulos et al (12) in Athens examined 23,850 men between 1977 to 1993 (17 years) and found similar results to Farjarinen (11).
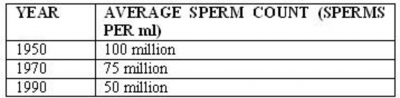
In Edinburgh a recent study by Irwin (2) saw a 25% decrease in sperm count over 20 years, the results are shown in table 1 below. The worrying thing about this downward trend is that a sperm count less than 20 million sperms per ml is interpreted as being infertile, if this downward trend of counts were to continue then values less than this will be the average in the next millennium.
Assuming, this data is correct, then the cause (or causes) must lie with changes in our environment or lifestyle over the past few years. We have released endocrine disrupters to our environment over time especially in Western industrialised countries where these horrific changes are most pronounced (2). Pharmacological investigations and natural poisoning episodes have led to the association between exogenous chemicals and alterations in multiple hormonal systems (3). Certain persistent environmental contaminants have been shown to modulate the activities of several different hormones. The unborn child or the neonate may be at special risk from these chemicals because of rapid growth and development, in addition to enhanced exposure to these persistent chemicals via food and water.
A possible explanation for this trend was put forward eight years ago by Sharpe
Normal Endocrine Control Of Testicular Sperm Production
I will firstly cover some salient points of the role of the testis in sperm production, especially the numerous feedback systems that exist. Before exploring the various postulated mechanisms responsible for the sperm count decrease over the years.
Sperm production in adulthood is limited by two factors, the efficiency (and normality) of the process of spermatogenesis and the number of Sertoli cells. These Sertoli cells orchestrate and control the process of spermatogenesis (2, 14 and 15), but each sertoli cell can only ‘nurse' a finite number of germ cells through development into spermatozoa (2, 6, 7, 14, 16 and 17). Hence the number of Sertoli cells present puts a ‘ceiling' on the maximum attainable germ cells being produced, and effects testicular size (6). In most mammals the number of Sertoli cells becomes ‘fixed' at a particular stage in development which varies between species (14).
In the rat Sertoli cell, multiplication commences at around 19-20 days of gestation and ceases at around day 15 of postnatal life, and thereafter no further increase in Sertoli cell number can be induced. Manipulating the endocrine environment prior to postnatal day 15 can alter Sertoli cell numbers. It has recently been found that suppression of the blood levels of follicle-stimulating hormone (FSH) in the critical period before day 15 inhibits Sertoli cell multiplication (3, 6, 14 and 18). If Sertoli cell multiplication is altered transiently, it results in adulthood with parallel reductions in Sertoli cell number, testicular weight and sperm output. The number of sperms produced is affected but the process of spermatogenesis remains normal. Spermatogenesis remains normal because there is little change in the cross-sectional appearance or size of the seminiferous tubules because Sertoli cell number affects primarily the length, not the breadth, of the tubules (6). Thus it can be said that FSH is probably the most important, though not necessarily the only, factor involved in regulating Sertoli cell multiplication (18).
Contrary to the above, in the neonatal rat, blood levels of FSH remain at a plateau up to day 15 and then increase dramatically beyond this time, i.e. just after Sertoli cell number has been ‘fixed' (14). The key to this appears to be a sudden change in negative feedback regulation of FSH by oestrogens acting on the foetal anterior pituitary gland beyond day 15 (2, 7 and 31). Sertoli cells from neonatal rats secrete oestrogens in response to FSH but between days 15 and 20 they lose the ability to make this steroid, which could be interpreted as a sign of Sertoli cell maturation (14). Thus, prior to postnatal day 15, oestrogens from the Sertoli cell keep blood levels of FSH in check whilst Sertoli cell number is being determined, this is a classic homeostatic feedback mechanism.
In turn FSH drives oestrogen production by the Sertoli cell, as well as increasing multiplication of Sertoli cells and probably regulating secretion of another hormone Mûllerian inhibiting substance (MIS), which causes the regression of Mûllerian ducts in the foetus (2 and 7). MIS may also be responsible for suppressing multiplication of germ cells during foetal life, because of its ability to inhibit growth of various cell types (7). However the limited data available for man (2 and 14) suggests that Sertoli cell number may increase not only during foetal and neonatal life, but also during puberty, so that the ‘window' for potential adverse effects on Sertoli cell multiplication may be longer than that in the rat.
Exposure of pregnant mice to oestrogen has also been shown to impair Leydig-cell development as well as reducing Sertoli cell number as outlined previously (7). Oestrogens negatively regulate Leydig-cell development via inhibition of Leydig precursor cell replication. This results in a decrease in testosterone production and thus reduced masculinisation in the animal, this is reflected by a reduction in sperm production (7). However oestrogen is found naturally within males, in the adrenal glands where a small amount of it is produced to act on receptor sites thereby producing a specific but unknown function (1).
So the effects of oestrogen on the testis and its relevance to this topic can be clearly seen. As a result of this a number of experiments demonstrating oestrogens effects on the testis have been carried out. For example Cook et al (19), like the majority of experimenters in this field, have consistently witnessed that in utero exposure to oestrogens will decrease Sertoli cell number and sperm production in a dose dependent manner. These effects have been accompanied by decreased testicular and epididymal weights, interstitial cell atrophy and seminiferous tubule degeneration. Moreover, immunisation of neonatal ram lambs against oestradiol, and especially oestrone, increases the rate of testicular growth with greater sperm production (14 and 21). It is presumed that these changes in testicular growth are at least partly a reflection of altered Sertoli cell number, and of particular significance is the suggestion that oestrone may have a special role to play in the feedback control of FSH secretion in this situation (21).
Oestrogens-Like Chemicals And Their Actions On Oestrogen-Receptors
During our everyday life we are continuously exposed to a cocktail of chemicals which can mimic the actions of the female hormone, oestrogen. Recent research has shown that many man made chemicals can act as weak oestrogens (xeno-oestrogens), mimicking in part the actions of our own natural hormones directly and indirectly (1, 2, 5, 14, 20, 30 and 31). These chemicals are present in the plastic lining of food cans, in pesticides, in plastics and in paints. In laboratories many designed chemicals have been shown to have oestrogenic effects.
Oestrogenic hormones exert their many effects by binding to intracellular oestrogen receptors, which consist principally of specialised proteins located within the target cells, they recognise the hormone and allow it to regulate specific oestrogen responsive genes within the cell. Oestrogen receptors allow many hundreds of different chemicals to bind to them. In some cases the chemicals have structures so dissimilar to that of the bodies natural oestradiol that they would never normally be thought of as having hormonal activity. These chemicals are very weak oestrogens (20, 30 and 31), but if given in high enough amounts they can activate oestrogen receptors in much the same way as natural hormones do (they do however bind to oestrogen receptors more weakly than the endogenous hormone). The presence of a phenolic hydroxyl group is a common feature of many oestrogenic compounds (30). For example, hydroxylation of
It was always thought that there was only one oestrogen receptor, however a second receptor (ERβ) has been identified which binds preferentially to certain environmental and natural oestrogens compared with the original oestrogen receptor (ERα). This new receptor is located in higher amounts in specific tissues in the body, such as the prostate and brain (20). Scientists now believe that this combination of different types of oestrogen receptor and differing tissue distribution may be crucial in determining if a particular part of the body is likely to be affected by natural or environmental oestrogens, for example the testes.
One hypothesis is that these ‘false' oestrogens latch on to these hormone receptor sites in the body, possibly blocking the action of the naturally occurring hormone. Another explanation is that the chemicals may mimic its action, switching on or turning off biochemical pathways, and hanging around in the body far longer than natural oestrogen (1). The result is potentially devastating effects on oestrogen-sensitive tissues in both sexes, particularly in the developing foetus.
Thus it can be seen that exposure to more than the normal level of oestrogen in the uterus at a critical period of foetal development could be responsible for these abnormalities being seen, especially the decreases in sperm counts.
Environmental Oestrogens
Professor Skakkebaek and Dr Sharpe have proposed many factors for increased oestrogen uptake and exposure since the Forties: dietary changes with increased consumption of hormone-rich dairy produce; synthetic oestrogens in the contraceptive Pill and other drugs, and a wide-range of chemicals that have been identified as having oestrogenic activity. Table 2 below shows the various routes of human exposure to oestrogens that have changed in the past 50 years. They include environmental contaminants such as DDT, PCBs (used in electronics), and exhaust fumes (1). So it can be seen that since the Forties man has produced and released into the environment increasing amounts of chemicals, some of which are highly persistent, present in our food chain and in our bodies (14 and 23) and which are weakly oestrogenic (24). Polychlorinated biphenyls are a good example, but several other chlorinated hydrocarbons (e.g. DDT) show similar properties. So it can be seen that humans now live in an environment that can be viewed as a virtual sea of oestrogens (7).
Researchers surveying the Great Lakes of Apopka Florida in the late Eighties found that members of 16 animal species, which fed on fish from the lakes (especially alligators and birds), were known to be contaminated with some sort of oestrogen-like chemical. These animals were failing to reach adulthood and were sterile (1, 5 and 30). Closer to home concerns about drinking water have been raised. Dr Jean Ginburg, a fertility specialist at the Royal Free Hospital in London, suggested that men in the Thames Water Authority area had poorer-quality sperm and were less fertile than men in other areas. However, Professor John Sumpter, a fish physiologist at Brunel University, says that drinking water is unlikely to be linked with a decrease in sperm counts “simply because we don't drink enough water” (1). He and his team are tracking the oestrogenic activity of effluent and are close to identifying chemicals responsible for the sexual alteration in fish near sewer outlets (2 and 11). The discovery of these hermaphrodite fish was kept quiet by the department of agriculture for 2 years! The team believes that the problem is not what you are drinking but what you are exposed to in your home. Michael Joffe another fertility expert, at St Mary's Hospital says “we still don't know what's causing falling male fertility, but many believe that oestrogen from women's urine in sewerage is the most likely contender” (2, 6 and 11).
Plastics
Boockfor et al carried out experiments injecting rats with a chemical called octylphenol (an organic compound based on a benzene ring), a decrease of FSH secretion in the foetus which consequently decreased Sertoli cell number and thus spermatozoa number was observed (11 and 20). Octylphenol is a breakdown product of a group of chemicals used in the manufacture of some detergents, plastics, textiles and paints. When the rats were given 80mg or 20mg of octylphenol twice a week, an accumulation of the chemical in tissues occurred, similar to that seen in fish from polluted rivers. After a month the rats given even the lowest doses of octylphenol showed reductions in sperm counts and fourfold increases in sperm abnormalities (11). It is still uncertain as to the extent to which the human body is actually exposed to chemicals such as octylphenol, and as yet there is no direct proof that there is any link between chemical exposure and changes in sperm counts in man.
Professor Soto in the US found that breast cancer cells were proliferating in his laboratory plastic dishes as though they were in the presence of oestrogen (2). He concluded that something in the dish was acting in a similar fashion to oestrogen. He found the agent to be nonyl-phenol, a widely used anti-oxidant, which is used widely as a spermicide foam so is presumed to be safe. The plastic lining of many tin cans and food wrapping contain lots of these plastics e.g. pthalates, and it has been found that these can leach into the contents, especially vegetables and fatty foods (2 and 6). Similarly for years dentists have widely used these sorts of agent in fillings. In these patients oestrogen like compounds have been found at high concentration in their saliva (2). Thus we are being exposed to and eating food-containing man made oestrogens all of the time unknowingly.
Therapeutic drugs
More evidence for these chemicals causing harm was demonstrated by the use of a drug known as DES (diethylstilbestrol), which was used widely in the livestock industry for 20-30 years (7), and for the first 20 years of their use it was not recognised that they might pose a risk to man. More importantly this drug was prescribed in high therapeutic doses to several million pregnant women worldwide for a variety of reasons such as to prevent miscarriage, between 1945 and 1971 (2, 3, 7, 14 and 30). It was a synthetic oestrogen manufactured to be orally active and resistant to degradation (structure in Fig 2.), so poses as a serious hazard to man. In 1970 genital abnormalities in the children of some of these women were subsequently linked to its use because they occurred at greater incidence than in normal controls. In boys the abnormalities included decreased semen volume and sperm counts, but there was no obvious abnormalities in the seminiferous tubules, interstitium or vasculature (6). Due to these observations Dr McLachlan in North Carolina exposed pregnant mice to DES during experiments in the early Nineties. He obtained hermaphrodites and sterile offspring with both male and female characteristics (1). Similarly boys born to mothers who conceived shortly after terminating use of the oral contraceptive pill, had an increased incidence of cryptorchidism (22) and decreased sperm production (6 and 14). Due to these observations in 1981 orally active anabolic oestrogens were banned in Europe and many of the anabolic oestrogens used now in the livestock industry are not orally active (7).
Other uses of synthetic oestrogens have increased in the past 20-40 years with increasing popularity of the oral contraceptive pill (e.g. ethinyl oestradiol). There are reports that ethinyl oestradiol is detectable in water sources but there is little data on concentrations in drinking water. However, like DES and other xeno-oestrogens, ethinyl oestradiol does not bind to sex-hormone-binding globulin (SHBG, to which most oestrogen in blood is normally bound), which means that it potentially has a very high biopotency if ingested (7 and 30).
Food sources
Certain plants and associated fungi are also sources of weak oestrogens (phyto-oestrogens) (3, 7, 25 and 30) and it is well established that in animals that there ingestion can grossly impair normal reproductive function (26). Shepherds have known for many years to keep their sheep out of certain kinds of clover or else the sheep become infertile. Soya products are particularly rich in phyto-oestrogens (7 and 27), and it is widely known that consumption of such products has increased considerably in the last two or three decades (14) as a substitute for meat protein. Table 2. shows that a change in diet over the last 50 years has had the greatest impact on increasing our oestrogen exposure. However exposure to phyto-oestrogens alone would probably be insufficient to induce major direct oestrogenic effects in most adults. Indeed, phyto-oestrogens may reduce exposure to endogenous oestrogens by stimulating production of SHBG by the liver and thus decreasing the concentration of bioavailable endogenous oestrogen (7), therefore protecting us.
A source of oestrogens (mainly oestrone sulphate) to which we are all exposed to is cow's milk (28). Pregnant dairy cows (which produce relatively high levels of oestrogens) continue to lactate (7) unlike humans, and their milk is processed along with that from non-pregnant cows for our consumption. It is not known whether the levels of oestrone sulphate in cow's milk have changed over the last 50 years as a result of alterations in farming practice (14), but the prevalence of bottle (as opposed to breast) feeding has increased considerably since the 1940's in most developed countries. Bottle milk is formulated from cow's milk (containing oestrone sulphate) whereas human (breast) milk contains negligible levels of oestrogens. Cows milk therefore contains substantial amounts of oestrogens and its consumption may lead to changes in oestrogen metabolism in man. Fortunately, it appears that the oestrone sulphate is removed during processing into formulate milk (7 and 29) so that this potential route of oestrogen exposure can probably be excluded, but it is still unclear whether the oestrogen might appear in other dairy products.
During mammalian lactation, many of the persistent hormonal contaminants, such as dioxins, PCBs, and DDT, concentrate in the milk, leading to lactational transfer to the nursing infant (6). Exposure to these lipophilic chemicals can be 10-40 times greater than the daily exposure of an adult (3). Although this may only occur for a short time, if this period corresponds with a sensitive developmental window, permanent alterations may result. Young children often eat a limited diet. If their dietary source is contaminated, greater relative exposure may occur for them than it would for an adult who eats a more varied diet.
Many of the atmospheric hormones are lipophilic chemicals and so are stored in adipose tissue (2, 3 and 30), chemical exposure therefore increases with age, due to the bioaccumulation potential of these substances. Weight loss may lead to minor reductions in the body levels of these chemicals. The relative consumption of fats (especially animal fats), proteins, and refined carbohydrates can substantially affect oestrogen excretion and metabolism. So the overall effect of eating a modern western diet, high in fat and low in fibre, is to increase exposure to endogenous oestrogens (2, 7 and 30). Treatments with agents that disrupt entero-hepatic recirculation or prevent uptake of fats into the body (e.g. high cholesterol drugs) have been reported to have some minor success in reducing body burdens. However, neither of these approaches has been shown to be very effective. The only process that does lead to significant depletion of stored amounts of these chemicals is lactation (3). However, lactation results in transfer of chemicals from the mother to the infant, who may be more sensitive to any adverse effects than the adult as previously discussed.
These days a higher proportion of farm animals are being fed hormones such as growth hormone, oestrogen and testosterone to increase their size and reproductive competence as increased protein to fat ratios result in increased yields e.g. cattle and “battery” hens. These hormones may be transferred across species barriers directly when we eat them or through our water supply therefore contributing to the declining fertility of man.
Insecticides, fungicides and pesticides
Although DES, Octylphenol, Phyto-oestrogens and milk demonstrate the potent effects of oestrogen like substances on human reproduction, none of them are proven environmental contaminants. As a result of this, insecticides such as Methoxychlor (6) and other pesticides that may be considered environmental oestrogens (30), including Kepone, dicofol and
Amongst pesticides, typical agents with oestrogen-mimetic properties include DDE (a major metabolite of DDT), atrazine, dieldrin, lindane, pentachlorophenol and toxaphene, have shown to decrease sperm counts in man according to Muller
Vinclozolin, a fungicide used on many types of fruits and vegetables, has been shown to be a potent anti-androgen, blocking the effects of the male sex hormones and resulting in demasculinisation of male offspring which is accompanied by a decrease in sperm count in the exposed (3).
Industrial chemicals
Dioxin (chlorinated hydrocarbon TCDD), the most toxic man-made chemical, is not an oestrogen-like compound (3,7 and 30), however, it can block the action of oestrogens under certain conditions and thus decrease Sertoli cell number. Exposure of pregnant animals to extremely low levels of dioxin (doses that do not adversely affect the mother) leads to alterations in the reproductive systems of the offspring. Many of the effects are not detectable until the offspring reach puberty. Sperm count is decreased in male offspring, and their mating behaviour is subtly altered.
PCBs represent a complex mixture of 209 unique compounds (structure in Fig 2.). Unlike dioxins, which are unwanted by-products of certain industrial processes and combustion, PCBs were commercially synthesised and used in transformers and capacitors. PCBs are resistant to thermal degradation and stability, which has led to their persistence and bioaccumulation in the environment (30). A small number of the PCBs are dioxinlike in their biological activity and can cause all of the effects mentioned above for dioxin. Other PCBs or their metabolites show oestrogenic properties.
The former Soviet Union's production of PCBs was only stopped in 1990 and although DDT production has been banned in the United States since the early 1970s, new factories are still being built to produce DDT in India (3). The presence of these chemicals in some developing countries is worrying since they are probably accumulating to harmful levels. Part of the concern about environmental hormones is that many of these chemicals do not readily disintegrate. Some persist in the environment for tens of years. It is therefore thought to be important to reduce additional input into the environment. But what do we do about the chemicals already in the environment?
These chemicals reach us through the atmosphere, where many of them bind to dust particles and are transported through the air, depositing on plants to enter the food chain, or landing on the water, where they eventually enter aquatic species or are drunk, to only bioaccumulate in man.
When animals are exposed to test chemicals, it is possible that toxic effects on other organs (e.g. the liver) other than the testis or reproductive axis could lead to a reduction in testicular size and thus daily sperm production as a result of non-specific effects (6). Christian and Gillies (31) carried out experiments to investigate the effects that environmental oestrogens had on hypothalamic dopaminergic neurone development. They found that development of these neurones was affected and stated that such actions could thus contribute to the effects which environmental chemicals have on the reproductive tract and reproductive behaviour. Although this possibility cannot be excluded completely, the present data provides little evidence for any such effect.
Changes in human exposure to oestrogens are difficult to quantify especially when the suspected alterations are in the metabolism/bioavailability of endogenous oestrogens during pregnancy. The most reliable (and safest) assumption is that pregnant women (and mankind in general) are exposed to more, rather than less, oestrogens than was the case 50 years ago. The extent of this increase, its source, and its consequences are likely to differ between countries and between individuals.
Other Factors That May Be Responsible For Decreasing Sperm Counts
Braun et al (33) have demonstrated that the levels of testosterone and oestrogen in stallions vary greatly depending which seasons it is. These hormones are at their lowest values during December. The relevance of this is that due to global warming, the seasons have and will continue to alter thus the levels of these hormones may consequently be varying and contributing to male sterility.
The increased temperatures in the world may be affecting normal sperm production, as it is known that testicles are anatomically positioned on the exterior of males because sperm production is highly temperature dependent, particularly spermatozoa which are susceptible to denaturation at high temperatures. However in countries with hot climates the theory is not supported as birth rates are relatively high, e.g. India and Africa, so this factor may be discounted.
With increasing ozone layer depletion due to the use of aerosols in the past and still in some countries, more radiation is reaching man. Exposure to radiation has further increased with the invention of televisions, microwaves, x-rays, nuclear weapons and the construction of power stations. It has been proven that radiation reduces sperm production in adult males (34). The effects of radiation on sperm production is more pronounced in children and the effects are seen at lower doses than those seen in adults.
Finally, many doctors believe the culprit of increasing male sterility may be the popularity of tight fitting underwear, but there is barley any evidence to support this “old wives tale” in the literature.
Summary
If the sperm count decrease is to stop, ultimately the causative agent/agents must be found or reduced. This is difficult because of the wide use of these chemicals worldwide. Another factor to consider is that the effects of these chemicals is additive, i.e. not one agent is to blame, thus the situation is complicated and there are no quick and easy solutions. The scale of the problem is overwhelming as a substantial amount of the global economy relies on these chemicals which are widely used in modern every-day life.
Many of the experiments cited above support the view that potential environmental endocrine disrupters should be examined at physiologically relevant concentrations, especially during development, because the majority of data obtained so far have been of supraphysiological therapeutic concentrations. As a result of this Congress has recently voted in an oestrogenic screening program (20) on all potentially oestrogenic sources, which is to be paid for by manufacturers themselves, who if they do not take part will have their products withdrawn from the shelves (2). However measures comparable to this are not being carried out in the UK, who seem to be undisturbed by the many reports and claim to be waiting for a report by the EU in the next few years.
We have facts and we have hypotheses on oestrogen exposure inducing male sterility, so it is important that we get together and find out the real answers. Thus researchers have a key role to play in understanding how these chemicals control the reproductive system, so that we are better able to judge if they really are a hazard to human health. Even with a variety, of different Government tests in place, human hazard assessment will be a challenging task. However collecting more information is our main priority because only then will we be in a position to assess the relationship between exposure and adverse effects and so see what chemical skeletons in our cupboards are likely to contribute to the increasing male infertility.
Nonetheless the culprit may not be oestrogenic molecules, but could be other changes over the years such as increased stress. The majority of studies that exist blame Xeno-oestrogens and so have attracted massive media attention (and probably lucrative research grants!). Thus these experimenters work may be biased and as already stated above they may be using unnaturally high doses of oestrogens in their studies to produce radical results, which may consequently be invalid.
However if the general fall in sperm counts are a consequence of altered oestrogen exposure in utero, then they are preventable. But because of human ignorance it seems that for the luxuries of modern life we will have to accept increased outbreaks of cancers and the possible extinction of the human race due to the decrease in fertility of our male species.