Bacteriological Quality of Water Samples of a Tertiary Care Medical Center Campus in North Western Himalayan Region of India
S Goel, R Sood, S Mazta, P Bansal, A Gupta
Keywords
mpn count, quality, retrospective study, water sample
Citation
S Goel, R Sood, S Mazta, P Bansal, A Gupta. Bacteriological Quality of Water Samples of a Tertiary Care Medical Center Campus in North Western Himalayan Region of India. The Internet Journal of Third World Medicine. 2006 Volume 5 Number 1.
Abstract
A total of 91 water samples were collected aseptically in sterilized containers from different drinking water sources of Medical College campus and field practice area of the department over a period of two years between March 2005 to February 2007. Bacteriological examination based on MPN count in 100 ml of sample revealed that 81.3% (89.7% in 2005-06 and 75% in 2006-07) samples did not meet WHO standards of quality of water samples. The samples taken from river, springs and bhowris were highly contaminated with MPN count more than 1600 in some cases. More samples collected from college campus were poorer in quality than collected from field practice area. The samples collected during summer and rainy seasons in 2005-06 were poorer in quality than collected during winter months. Thus present study suggests that surface and ground water samples in and around Dr. R.P Govt. Medical College campus, Kangra are highly contaminated with feacal material, which may further lead to outbreaks of gastrointestinal diseases. Therefore, routine monitoring of water purification system should be done by various related departments like IPH, PWD and Community Medicine.
Background
The 700 bedded Government Medical College, Kangra at Tanda, Himachal Pradesh was conceived in 1997, and includes one Hospital situated at Dharamshala, around 19 KMs away from Medical College campus. In medical college campus, a total of around 300 families reside and 250 MBBS students study their undergraduate course. Besides this, there are around 500 employees working in the hospital.
At the inception of Medical College at Kangra, water supply to the college campus was solely maintained by five ground bores dug deep in the earth. Later this system could not cater the need of increasing residents of the campus. The department of Community Medicine with a water testing laboratory was later established in 2002. Some samples of water supplied to medical college campus were tested on pilot basis to test the quality of water supplied to campus residents and employees of medical college. The report was an eye opener as it was observed that Most Probable Number (MPN) of the water samples collected was in range of 500 to 2000. This was enough to cause water borne outbreaks in the campus. In the mean time, an outbreak of Jaundice spread among MBBS hostel students which led to an immediate joint inspection of water system and water supply scheme. It was led by Principal Medical College and associated with Departments of Community Medicine, Microbiology, PWD and Irrigation and Public Health (IPH). It was observed that water coming out for supply to residential premises of college campus was turbid to naked eye. On enquiry, it was revealed that steps of purification were not being followed rigorously. There was undue stress on chlorination and the essential basic steps of chemical mixing, flocculation and sedimentation and filtration was not being followed before chlorination. This led to ineffective chlorination of water supply. It was also noticed that there was lot of animal and human trespassing along with defecation surrounding the source of water supply.
Therefore, a decision was taken by Department of Community Medicine to regularly monitor the quality of water samples in the vicinity of the medical college campus area. For this, a senior laboratory technician was trained in collection and testing of water samples on every 10 th , 20 th and 30 th of the every month. Keeping in mind increasing trend of gastroenteritis and outbreaks of gastrointestinal diseases in different parts of Himachal Pradesh as well as campus of medical college, present study was envisaged with following aims and objectives.
Aims and Objectives
-
To study the water bacteriology of samples collected from Medical College campus and field practice area of the department
-
To assess the seasonal variations of coliform bacteria in these resources
-
To advocate public health authorities regarding measures to be taken for poor quality of water samples
Material and Methods
Few informal visits were made to water treatment plant along with students of MBBS class between March 2005 to April 2007 for observation of storage, filtration, and disinfection process.
Besides this, retrospective data of collected water samples between March 2005 to February 2007 was retrieved from the records/ registers maintained in laboratory of department of Community Medicine, Dr RP Government Medical College, Kangra. A total of 91 water samples (39 between March 2005 to February 2006 and 52 between March 2006 to February 2007) were collected from Medical College campus and field practice area of the department over a period of two years (w.e.f. March 2005 to February 2007). The samples were collected aseptically in sterilized containers and tested by a trained health educators and senior laboratory technician of the department by a predefined laid down guidelines by WHO and ICMR in ‘WHO guidelines for Drinking Water Quality 2 ' and ‘Manual of Standards of Quality for Drinking Water Supplies' 3 .
Diagnostic Criteria- Two hundred milliliters of water samples from each source were collected in sterile glass stoppered bottles for microbiological examination. The samples were transported and stored strictly in accordance with guidelines described in standards methods. Presumptive coliform count test based on multiple tube fermentation method by Senior BW 4 was used to estimate the most probable number (MPN) of coliform organism in 100 ml of water for diagnosis of bacteriological contamination. The test was carried out by inoculation (for 48 hours at 35°C) of measured quantities of sample water (0.1, 1.0, 10, 50 ml) into tubes of double and single strength McConkey's Lactose Bile Salt Broth with Bromocresol purple as an indicator. The tubes showing gas formation were regarded as ‘Presumptive Coliform Positive'. The results of MPN are interpreted by McCardy's probability tables from the no. of tubes showing acid and gas (fermentation by coliform organisms) 5,6 . International WHO standards which recommend not more than 10 coliform per 100ml in un-piped rural supplies and not greater than 3 per 100ml. in non- chlorinated pipe supplies 11 (but not in repeated samples) was used in the study to define the sample as satisfactory or unsatisfactory .
Results
During informal inspection of water treatment plant, following things were observed regarding storage, filtration and disinfection.
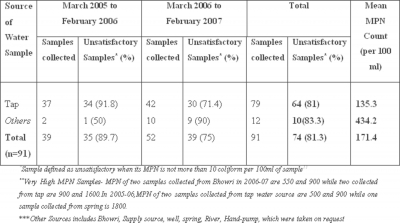
Out of a total of 91 samples collected over a period of two years, 79 (86.8%) were collected from taps, 7 (7.6%) from
During the year 2005-06, 89.7 % samples were found to be unsatisfactory (bacteriologically non-portable) while 75% samples were reported unsatisfactory in 2006-07. Majority of the samples were collected from taps, from which around 81% were found unsatisfactory. Most of the samples (83.3%) taken from other drinking sources (
Figure 1
As evident from Table-2, the samples shows significantly lower coliform count during peaks of summer seasons as compared to other seasons. A higher coliform count (89% of samples collected over a period of two years) was recorded during rainy seasons, which decreased during winter seasons (71% of samples collected over a period of two years) and again increased during summer seasons. The average coliform count recorded in rainy seasons was 288.3 as compared to winters (96.8) and summer season (56.8).
Discussion
Our results are consistent with other studies mentioned in the paper in same settings as well as different settings.
Much of the ill-health which affects humanity, especially in developing countries can be traced to lack of safe and wholesome water supply. There can be no state of positive health and well being with-out safe water. Since water is vital for our life, we expect it to be clean and safe. Even water that appears problem free may not necessarily be safe or acceptable. The water intended for human consumption must be free of pathogenic and chemical agents, pleasant to taste and usable for domestic purposes. Since water is the most important potential source of infectious diseases so water purification is the most important potential single measure available for ensuring public health.
Himachal Pradesh is located in north-western Himalayas and is rich in water resources. In recent years, these resources are being contaminated by increased human activities. Studies by various researchers have pointed out various quality issues in water supplied. According to a survey conducted by the Himachal Pradesh Water and Sanitation Mission, 70 to 80 per cent of endemic diseases in the state were water borne. They were diarrhea, dysentery, typhoid, jaundices and cholera etc. The survey report also revealed that every year several deaths particularly among children occurred due to water-born diseases. This was because of deteriorating quality of drinking water. The report quoting WHO said that more people, in fact would die of consuming unsafe drinking water and unsanitary conditions by the year 2020 than from AIDS, if steps to improve water quality were not taken on war footing 1 . The Swajaldhara Yojna under Rajeev Gandhi National Drinking Water Mission, Sanitation campaign and Water Quality Monitoring and Surveillance is being initiated in the state.
For ensuring safe and portable water supply, water should pass through three stages- Storage, Filtration and Disinfection. Storage removes 90-95% of the physical impurities by mechanism of suspension. It also allows penetration of light which results in oxidation of organic matter by aerobic bacteria, thus decreasing free ammonia content of water. It also decreases total bacterial count by as much as 90 percent. Filtration is second stage of water purification which results in 98-99% drop in bacterial count apart from other impurities. Disinfection is final stage of purification which results in destroying all pathogenic organisms left after storage and filtration. All these stages are required in series for purification of water. Even if one stage is by-passed, the water may not be rendered fit for drinking purposes.
The contaminated waters should be treated before public distribution to reduce contaminants and thus making it suitable for human consumption 7 . The report of the year is defined as satisfactory when 8
-
More than 95% of the samples should not have any coliform bacteria.
-
No two consecutive samples should have coliform bacteria
-
No sample should have E.Coli.
In our study, the indicator organisms namely heterotrophic bacteria, total and feacal coliform were assessed for ensuring water quality, which was also taken as one of the indicator of water quality in other studies [[[ 8a]]] . Coliform organism has long been recognized as a suitable microbial indicator of drinking water quality largely because they are easy to detect and enumerate in water. If coliform bacteria are detected in more than 95% of samples, then it indicates feacal pollution. It was observed in our study that more than 80% of the samples taken over a period of two years were found to bacteriologically contaminated with total coliform count ranging from 40 to more than 1800. Heavy coliform count detected in our samples shows that surface water is highly contaminated with animal or human fecal material. Actually in this part of the country, livestock is kept on open grazing system on hill slopes. During rains their feces flow to rivulets with rain water. In addition to it , large pasture area have been submerged in water reservoirs of mega-hydroelectric project leading to increase pressure on limited pastures as well as to keep the forest cover in controlled limits, plant like eucalyptus have been planted on lower Shivalik ranges which in turn have resulted in poor grass development beneath them. Thus, migratory flocks too make early movement to alpine pastures and do not give enough time for proper growth of alpine pastures. This also leads to heavy contamination of water resources. Wild fauna is also another important source of contaminant. Lastly but not the least the population pressure cannot be ignored as an important source of contamination. Migrant workers engaged in construction in and around the campus do not have facility of sanitary toilets, thus forced to defecate in open leading to contamination of water resources. Sinha (1991) et al has also reported number of coliform bacteria which varied from 600-1600/100 ml from Susta pond and 1200-2000/100ml from Madhaul pond of Muzzarpur, Bihar 9 . Similarly 197 coliform species have been isolated from drinking water in five rural areas of Lucknow 10 .Whereas international standards recommend not more than 10 coliform per 100ml in un-piped rural supplies and not greater than 3 per 100ml. in non- chlorinated pipe supplies but not in repeated samples 11 . In the annual report of 2002 NICD Connoor have reported MPN coliform counts/ values above > 10 per 100ml in 22 taps out of 56 taps exclusive in Metlupalaiyam 12 .
In our study, a significantly high level of contamination was observed in samples collected from
It was further observed in our study that MPN of all the water sources were higher in rainy
The present study suggests that surface and ground water samples in and around Dr. R.P Govt. Medical College campus, Kangra are highly contaminated with feacal material, which may further lead to outbreaks of gastrointestinal diseases. In the state of HP, Irrigation and public health (IPH) department is managing the drinking water schemes and supplying the water to the household. The emphasis hitherto had been on adequate (quantity) supply of piped water, with little attention to quality. Although IPH labs usually certify the water quality regularly, an additional independent monitoring mechanism of quality of drinking water supply by Department of microbiology or Community Medicine needs to be done on a regular basis with a system of feedback and corrective action. It should not be just a knee jerk response after being highlighted by the media. It is further to be stressed that rapid sand filters needs more technical skills to operate and maintain for good quality filtration. Therefore, the system of imparting pre-service and on -the -job trainings to the personals employed with IPH for maintaining quality water supply should be strengthened. Further, as MPN count is relatively simpler and sensitive test for detection of coliform bacteria and henceforth indicator of water quality, this can be used at sub-center levels after imparting adequate basic training to health worker.
Recommendations
-
Cleaning of flocculator and sedimentation tank should be done periodically (at least once a week). This will ensure supply of clear water for chlorination, which will reduce chlorine demand and render chlorination effective. Similar regular (at least weekly, more frequently depending on loss of head and turbidity of water) back-washing of rapid sand filters should be done to dislodge the impurities from sand bed. Fencing of reservoir area should be done to restrict the entry of animals and persons who defecate around it. The protection of the area should be done in consultation with IPH, Local administration and Public Health specialist.
-
The prerequisite purification steps of storage, filtration and disinfection should be adopted religiously without by-passing of either step.
-
The main supply source should be regularly chlorinated as per guidelines, especially in monsoon seasons, when it should be super-chlorinated. Chlorine demand of the water should be routinely estimated for measuring the amount of chlorine to be added to water. The minimal period of contact between chlorine and constituents of water should be one hour. The rapidity of disinfection with chlorine is directly proportional to temperature of water, and inversely proportional to turbidity in water. Regular orthotouline test should be done to verify the required amount of chlorine in water. One additional chlorination apparatus should be kept for use during breakdown period.
-
The turbidity and color of the water after the filtration process should be assessed before the addition of chlorine. For optimal effect of disinfection with chlorination, water should be free from turbidity(less than one unit), color (less than one unit) and taste.
-
Records of cleaning of storage tanks/ filters, backwashing of rapid sand filters, and chlorination should be maintained. Similarly, records of loss of head and results of orthotouline test should be recorded. Chlorine demand of water should also be assessed at least monthly (more frequently in rainy seasons) and recorded.
-
The sampling of water samples from hostel mess and other eating joints should be done more frequently (at least weekly) in monsoon seasons. Samples of water should be taken systematically by a well laid out plan for a year from supply source, households and other major sources of water supply.
-
Regular monitoring of water supply system along with joints in the pipes for leakage should be done by IPH Department. It should be ensured that there is no back-siphoning while distribution of water from reservoir. Monitoring of water purification system must be done thrice in a year by a joint team comprising of Department of Community Medicine, IPH and PWD departments. Once a month monitoring should also be done independently by IPH other than thrice a month already been carried out by the Department of Community Medicine
-
As a short term measure, till the quality of water supplied improves, household level purification is recommended as a measure of personal protection.
Correspondence to
Dr Sonu Goel, Assistant Professor
Dept. of Community Medicine
Dr RP Govt. Medical College,
Kangra at Tanda, Himachal Pradesh, India
Ph-91-9418039835