Identification of Biomarkers in an Oral Malignant Melanoma Case with Potential for Therapeutic Intervention
R Pai, S Pai, R Lalitha, S Kumaraswamy, N Lalitha, R Johnston, M Bhargava
Keywords
carcinoembryonic antigen, malignant melanoma, myc, oral cancer
Citation
R Pai, S Pai, R Lalitha, S Kumaraswamy, N Lalitha, R Johnston, M Bhargava. Identification of Biomarkers in an Oral Malignant Melanoma Case with Potential for Therapeutic Intervention. The Internet Journal of Oncology. 2009 Volume 7 Number 1.
Abstract
A 32-year old female patient presented with the tumor of the oral cavity at the Kidwai Memorial Institute of Oncology, Bangalore, India. Clinical examination and histopathological analyses confirmed the neoplasm as malignant melanoma. This rare melanoma of the buccal mucosa expressed carcinoembryonic antigen (CEA) as well as contained cells that expressed elevated levels of the c-myc oncogene product as identified by immunohistochemistry of the tumor biopsy. Presence of these tumor makers in the rare case of malignant melanoma with poor prognosis should allow exploration of novel targeted therapy against CEA and c-Myc.
Introduction
In South Indian population, prevalence of long term chewing habits of betel leaves, areca nut and tobacco culminates in the genesis of the neoplasms of the oral cavity in a large fraction of the habitual users1, 2. Majority of head and neck cancers presented at our institution are oral squamous cell carcinoma (OSCC). However, occasionally cases of malignant melanomas are encountered in the oral cavity. Oral malignant melanoma is a rare entity occurring at a frequency of less than 2% of the all the head and neck cancers3. When melanocytes or their precursor cells become neoplastic, they lead to malignancies which usually metastasize to adjacent tissues4. Hence, malignant melanomas are difficult to treat with poor prognosis. They are also known to be aggressive tumors and spread to tissues adjacent to the primary tumor and are resistant to radiation and chemotherapy. Treatments of melanomas which are refractory to conventional modalities of therapy pose a problem to clinicians. Hence, novel modalities of treatment such as immunotherapy and anti-sense therapy need to be explored to combat the disease. One of the targets to be considered for successful treatment of such tumors could be the carcinoembyonic antigen (CEA) against which immunotherapies are being developed5, 6. Further, studies on biomarkers such as CEA would be of immense benefit for diagnosis as well as prognosis of the disease. Effective management could also be achieved if successful antisense therapies against key oncogenes such as c-Myc are developed, which is an approach that is being pursued by several researchers7-9. Therefore, identification of tumor markers such as CEA and c-Myc in this form of malignancy could be very beneficial in treating this malady.
Case Report
Examination of the Level 2, 3 nodes showed metastases whereas level 4,5 nodes were free of the tumor. Histopathological analyses confirmed the tumor to be malignant melanoma. Microscopic sections revealed epitheloid pigmented cells arranged in nests, invading the underlying connective tissue. Focal areas showed atypical, pigmented spindle shaped cells with fascicular arrangement. Cells showed clear cytoplasm with large nuclei and prominent nucleoli. Few cells showed pleomorphism and nuclear hyperchromatism. Sparse inflammatory infiltrate was seen in connective tissue.
Figure 2
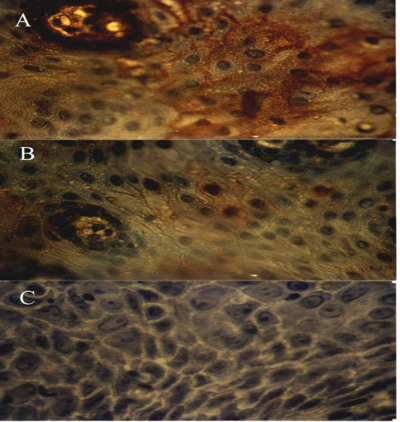
Immunohistochemical characterization of the tumor tissue sections were carried out for identification of CEA. The tumor cells were positive for anti-CEA reactivity suggesting the expression of the biomarker CEA by the melanoma cells (Fig.2A). When the procedure was employed using anti-Myc antibodies, positive staining was observed in a few cells in the tumor (Figure 2B). Immunostaining of tumor tissue sections performed by omission of the antibodies in the assay showed no staining indicating the specificity of the reaction (Fig. 2C).
Discussion
Initiation of oral neoplasia in the South Indian population is contributed to a large extent due the betel chewing habits. The physical insult of retaining the quid in the oral cavity for extended periods of time coupled to the interaction of the chemical components results in the neoplastic progression. Even though majority of the tumors are OSCC, rare cases of malignant melanomas are presented. These are of interest due to their location, aggressive nature as well as difficulty in clinical management by conventional modalities of treatment resulting in poor prognosis. Hence, novel treatment protocols are warranted to successfully manage oral malignant melanomas. To this end, we investigated the expression of CEA and c-Myc in a rare case of oral malignant melanoma.
CEA is expressed in a large number of tumors including the malignant melanomas10-12 and its presence is known to contribute towards an aberrant phenotype in some melanomas13. M 21 melanoma cells derived from xenografts have also been shown to express CEA14. Duray et al, detected CEA in primary as well as metastatic melanomas using monoclonal antibodies15. Elevated expression of CEA has also been documented in metastatic melanoma of cutaneous origin16. Other head and neck cancers such as submandibular gland carcinomas are also known to express CEA11. CEA is also considered as an important circulating biomarker in oral cancers17.
Another important biomarker in melanomas is the oncoprotein c-Myc. Increase in the expression of the c-Myc oncogene is known to be a hallmark of enhanced proliferation potential. Observations from cutaneous malignant melanoma exhibited an increase in mitotic rate with concomitant increase in c-Myc expression18. Moderate increases in the amounts of the oncogene c-Myc in metastatic melanomas has also been reported16.
The rarity of oral melanomas and poor prognosis necessitates characterization of this class of neoplasms for efficacious management. To the best of our knowledge, this is the first report of a malignant melanoma of the oral cavity which expresses CEA identified by immunohistochemistry. We have recently reported the expression of CEA in OSCC19 and it is interesting to note that the rare malignant melanoma we studied also share this property.
We could also identify a few cells that express c-Myc in this malignant melanoma from the palate region. These cells could be the cells which have increased mitotic rate as described by other investigators in cutaneous melanoma cases18. The c-Myc protein is suggested to be a significant contributor for malignant progression of melanomas20 and the over-expression of this oncogene leads to poor prognosis for this class of tumors21. In addition, c-Myc is considered an independent prognostic marker in the case of primary melanomas22 and in metastatic melanomas23. This oncoprotein is known to be associated with the development of neoplastic transformation in oral cancers as well24. Melanomas of the oral cavity are not only rare types of tumors but are also aggressive and have a great potential to metastasize especially exhibiting hematogenous invasion and have poor prognosis when compared to the cutaneous melanomas. Antibody mediated therapy especially against CEA may be important in designing efficient treatment protocols. Several immunotherapeutic approaches using CEA are currently underway and successful clinical trials of these entities should hold promise for employing these strategies against CEA-expressing oral malignant melanomas6, 25, 26. While other therapeutic options are also being explored, the down regulation of c-Myc using liposomal c-Myc antisense oligonucleotide which is known to inhibit the growth and metastases of human melanomas as well as combination of c-Myc therapy and conventional chemotherapeutic agents such as doxorubicin could also lead to efficient treatment modalities for oral malignant lesions8, 26.
Acknowledgements
The study was supported by the Indian Council of Medical Research, New Delhi, India.