Thrombocytosis and Gemcitabine
D Rossi, P Alessandroni, S Fedeli, P Giordani, V Catalano, A Baldelli, A Fedeli, G Catalano
Citation
D Rossi, P Alessandroni, S Fedeli, P Giordani, V Catalano, A Baldelli, A Fedeli, G Catalano. Thrombocytosis and Gemcitabine. The Internet Journal of Oncology. 2001 Volume 1 Number 2.
Abstract
Gemcitabine (2'-2'-difluorodeoxycytidine, dFdC) is a novel nucleoside analogue of deoxycytidine recently introduced for the treatment of pancreatic cancer and non-small-cell-lung cancer (NSCLC). We studied the occurrence of thrombocytosis when using this drug.
Introduction
Gemcitabine (2'-2'-difluorodeoxycytidine, dFdC) is a novel nucleoside analogue of deoxycytidine recently introduced for the treatment of pancreatic cancer and non-small-cell-lung cancer (NSCLC). Gemcitabine is inactive in parental form but is progressively phosphorylated intracellularly to its active diphosphate and triphosphate metabolites. The diphosphate inhibits ribonucleotide reductase (Heinemann et al, 1990) and the triphosphate is incorporated into DNA as a fraudulent base in competition with dCTP (Huang et al, 1991). After incorporation into DNA, one further nucleoside is added before DNA synthesis is terminated ("masked chain termination"). This appears to prevent excision repair of the altered DNA sequence and thus overcome one important potential resistance mechanism (1). Gemcitabine is commonly administered on a weekly schedule at the dose of 1000 mg/m2 on day 1, 8, 15 of a 28-days cycle. Phase II studies in NSCLC demonstrated response rates of 20% with relevant symptomatic benefit (2).
Review
In pancreatic cancer, randomized studies have shown superiority of gemcitabine compared with 5-fluorouracil in terms of clinical benefit, time to progression and survival (3). Gemcitabine has been tested in these last years in a number of solid tumors such as breast, bladder, ovarian, head and neck cancers. In breast cancer, response rates were 29% in anthracycline-resistant disease (Spielmann ET al, 1996) and 46% as first line chemotherapy for advanced disease (Blackstein ET al, 1996). Activity of gemcitabine in ovarian cancer ranged from 13% to 24% of response rates (4). In bladder cancer, response rates up to 38% were reported in chemo-naive patients (5) and modest activity has been documented even in head-neck cancer (13%; Catimel G et al, 1994) and small cell lung cancer (27%; Cormier et al, 1994). Activity has been reported even in T-cell-lymphomas (Zinzani et al, 1998).
Side Effects
The main side effects are neutropenia (26%) and thrombocytopenia (5-10%). Other toxicities include: nausea and vomiting, skin reactions, reversible increase of liver enzymes, proteinuria and hematuria, peripheral edema and flu-like syndrome. The drug information leaflet describes the possibility of thrombocytosis, which may occur in 7% of patients. However, we think that this "side effect" is underestimated and that it happens more frequently than has been documented until to day.
Thrombocytosis
A Medline search did not show any reference. However, we performed a phase II study of cisplatin and gemcitabine in patients with locally-advanced and metastatic non-small-cell-lung-cancer (gemcitabine 1000 mg/m2 on day 1 and 8; cisplatin 80 mg/m2 on day 2 every 28-day; one-hundred-twenty cycles were delivered with a median of 3 per patient).
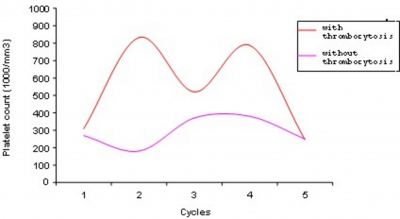
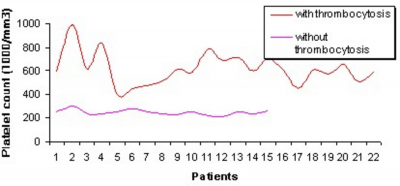
Thirty-seven patients were assessable for toxicity and 22 of them (59.4%) had thrombocytosis with platelet counts ranging from 401,000/mm3 to 992,000/mm3 (Table 1). The highest platelet increase was documented after the first cycle in 16 of 22 patients (72.7%) just one week before day 1 of the second cycle or a few days after. The platelet count normalized at the end of chemotherapy and neither thrombotic nor ischemic cardiovascular episodes were registered. In 2 patients with a platelet count greater than 900,000/mm3, thrombocytosis persisted for one month, and acetylsalicylic acid (100 mg once a day) was administered to prevent possible stroke or coronary thrombosis (Fig. 1 and 2).
Figure 2
Figure 3
The mechanism that induced platelet increase especially during the first cycle in these patients has not been elucidated; it could simply be a rebound with excessive platelets formation after the nadir and it is possible that the combination of cisplatin could play an important role.
Prolonged thrombocytosis could be deleterious in patients with cardiovascular disorders and cardiopathy for whom any platelet increase could cause undesirable complications. It would be interesting to revisit other trials of gemcitabine to confirm the real value of this observed side effect.
Correspondence to
David Rossi MD Azienda Ospedaliera "Osp. S. Salvatore" Via Lombroso 61100, Pesaro Fax (+ 39) 0721/364094 E-Mail: D.Rossi@Ospedalesansalvatore.It