Drugs Which May Exacerbate or Induce Myasthenia Gravis: A Clinician's Guide
A Ahmed, Z Simmons
Keywords
adverse drug reactions, myasthenia gravis, pharmacotherapy, therapeutic
Citation
A Ahmed, Z Simmons. Drugs Which May Exacerbate or Induce Myasthenia Gravis: A Clinician's Guide. The Internet Journal of Neurology. 2008 Volume 10 Number 2.
Abstract
When a patient develops an exacerbation of myasthenia gravis, the treating physician searches for possible causes. One such cause is the prescription of one or more medications which may worsen neuromuscular transmission. Clinicians should be aware not only of the specific medications which may produce such exacerbations, but also of the probability of exacerbations with specific medications, so that they can balance the potential for good and the potential for harm in order to arrive at a reasonable therapeutic plan. An awareness of medications which may trigger myasthenia gravis in patients with no history of this disorder is important as well, particularly for neurologists who are asked to evaluate such patients. For those patients with myasthenia gravis undergoing anesthesia, anesthesiologists should be aware of anesthetic agents which warrant special attention.
Introduction
Myasthenia gravis is an immune-mediated post-synaptic disorder of neuromuscular transmission, most commonly presenting as oculobulbar and proximal muscle weakness associated with easy fatigability. Because a variety of pharmacologic agents may affect neuromuscular transmission, physicians caring for these patients often must determine whether the medications needed by these patients are likely to adversely affect their myasthenia gravis. This is a particularly important question when such medications are being prescribed for potentially life-threatening conditions such as cardiac arrhythmias, or when anesthetic agents must be chosen for life-saving surgery. In addition, many patients with myasthenia gravis are treated with immunosuppressive agents, leading to more frequent and serious infections than are seen in the general population, and requiring judicious choices of antibiotics. The purpose of this review is to provide a clinician's guide to medications which may exacerbate myasthenia gravis, or which may produce signs and symptoms of myasthenia gravis in patients without a known defect of neuromuscular transmission. We also call attention to special considerations regarding the use of anesthetic agents in these patients. This is not intended as a comprehensive review of pharmacologic mechanisms, but rather as a practical, concise summary of drugs which should be of use to specialists and generalists alike.
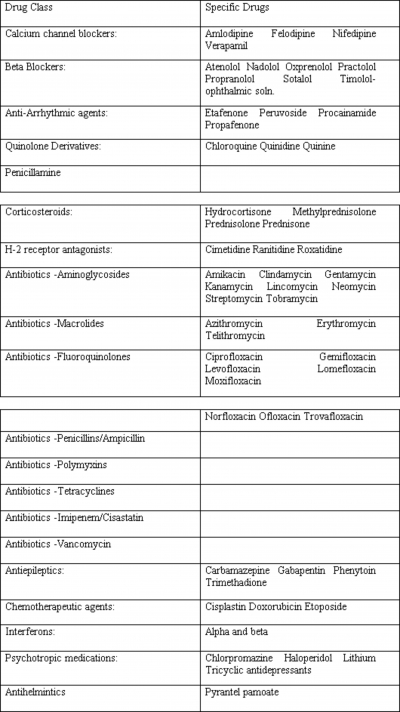
Calcium Channel Blockers
The mechanism by which calcium channel blockers affect neuromuscular transmission is not well established. They are believed to act at both the presynaptic and postsynaptic levels via blockade of L-type calcium channels. 1,2 Verapamil also prevents potassium outflow at the motor end plate and causes a decrease in intracellular ionized calcium levels. 3 In patients without known defects of neuromuscular transmission, the calcium channel blockers felodipine and nifedipine have been found to produce features of myasthenia gravis including dysphagia, ptosis, and generalized weakness after long-term use ranging from 18 months to 12 years. These patients were seronegative for acetylcholine receptor antibodies but demonstrated decremental responses on repetitive nerve stimulation. Discontinuing these drugs resulted in resolution of their symptoms, which re-appeared after the medications were reintroduced. 1 Similarly, oral use of verapamil and amlodipine for at least 3 months in patients with no prior known neuromuscular junction defects has resulted in abnormal jitter on single-fiber EMG study. 2 In patients with known myasthenia gravis, nifedipine caused worsening of symptoms. 4 In contrast, short term use of verapamil did not worsen the decrement seen on repetitive nerve stimulation when given to patients with myasthenia gravis as a single injection or when given orally for 14 days. 5,6 Thus, although not absolutely contraindicated, calcium channel blockers should be used with caution in patients with myasthenia gravis. Short term use appears to be less risky than long term use.
Beta Blockers
Case reports describe a number of beta blockers , including propranolol, practolol, oxprenolol, atenolol, sotalol, nadolol, and ophthalmic timolol which appear to have produced symptoms and signs of myasthenia gravis in patients with no known defects of neuromuscular transmission, or to have exacerbated myasthenic symptoms in patients with known myasthenia gravis. 7,8,9,10,11 Findings have included ptosis, diplopia, and decremental responses on repetitive stimulation studies. The mechanism may be at the level of the neuromuscular junction or the muscle membrane. 7,8 Recovery has followed discontinuation of these medications. 7,8,10,11 Doses have varied widely, and no clear dose-response relationships with severity of symptoms of myasthenia gravis have been established. As with calcium channel blockers, short term use of propranolol intravenously as a single dose, or orally for 14 days has not been found to worsen the decremental response on repetitive stimulation in patients with known myasthenia gravis. 5,6 We believe that chronic use of beta blockers must be undertaken with great caution in patients with myasthenia gravis, whereas short-term use appears to be less risky.
Anti-Arrhythmic Agents
Anti-arrhythmic agents including procainamide, etafenone, peruvoside, and propafenone have been reported to both induce and exacerbate myasthenia gravis. Descriptions consist primarily of case reports 12,13,14,15 . Procainamide is believed to act both pre-synaptically and post-synaptically, 12,13,16 decreasing the release of. acetylcholine 12 and reducing the susceptibility of the muscle membrane to action potentials. 12,13 Propafenone and procainamide appear to act as sodium influx blockers. 12,15 Myasthenic symptoms were seen with acute and chronic use; the duration of treatment ranging from a few hours to 8 months before development of symptoms. Drug levels and dosages varied, as with beta blockers. Caution and close observation are needed if these medications must be used for short-term or long-term therapy in patients with myasthenia gravis.
Quinolone Derivatives
Quinine, quinidine, and chloroquine affect neuromuscular transmission both presynaptically and post synaptically. 17,18 At the presynaptic level there is a reduction of acetylcholine release due to blockade of voltage dependent sodium channels, 18 and postsynaptically there is potentiation of depolarization. 17 Some patients with no evidence of a pre-existing neuromuscular junction disorder developed ptosis, diplopia, dysarthria, dysphagia, and generalized weakness while taking chloroquine. 19,20,21 Single-fiber EMG study or repetitive stimulation studies were abnormal. Clinical and electrodiagnostic abnormalities resolved with discontinuation of the medication. Some reports have found that acetylcholine receptor antibodies were absent in such patients. 17,19,21 Others have noted these and other antibodies such as anti-DNA and anti nuclear antibodies to be present, but to disappear after discontinuation of these medications. 20 Thus, quinolone derivatives, like many of the medications discussed here, must be used with caution in patients with known myasthenia gravis. In addition, the treating physician should be alert to the possibility of the development of symptoms of myasthenia gravis in patients treated with these medications who have no known history of this condition.
Penicillamine
Penicillamine is used for treatment of a variety of autoimmune disorders, and may be associated with the development of myasthenic symptoms including diplopia, dysarthria, and ptosis, in association with elevated titers of acetylcholine receptor antibodies. 22 Multiple mechanisms are thought to contribute to development of myasthenia. Some of these patients may have autoimmune-mediated subclinical defects of neuromuscular transmission which are unmasked by penicillamine. 3,23,24,25,26 Another potential mechanism is a direct immunomodulating effect of the drug by binding to the acetylcholine receptors with resultant production of antibodies directed against the receptors. 27 Prostaglandin E1 induced muscle weakness has also been postulated. 28 Generally complete resolution of these symptoms and disappearance of acetylcholine receptor antibodies occurs with discontinuation of the penicillamine. 27,29,30,31 Most reports of penicillamine-associated myasthenia gravis involve long term therapy, typically 2 months to 2 years. 27,30 Penicillamine can be used with caution and close monitoring in patients with myasthenia gravis.
Corticosteroids
Corticosteroids are considered to be effective treatment for myasthenia gravis, due to their immunosuppressive action which includes decreasing lymphocyte activation and migration. 32,33,34 However, steroids can worsen muscle strength acutely, 35,36,37,38 likely due to a direct blocking effect on the acetylcholine receptor through ionic channels 39 or an effect on muscle contractility. 39 Decreased protein synthesis and myosin loss have been identified in patients who weaken with steroids. 40 Steroid-induced exacerbations of myasthenia gravis are more likely in older patients with bulbar symptoms. 34 With long term use, steroid myopathy may result. 33
H-2 Receptor Antagonists
Animal studies report neuromuscular transmission abnormalities with H-2 receptor blockers such as cimetidine, ranitidine, and roxatidine. 41,42,43 Postulated mechanisms include pre-synaptic and post-synaptic interactions 43 and inhibition of acetylcholinesterase, 43 however there are no convincing reports of induction or exacerbation of myasthenia gravis in humans. Caution should be exercised when using these agents in individuals with myasthenia gravis, but they are not contra-indicated.
Antibiotics
Both induction and exacerbation of myasthenia gravis can occur with the use of some antibiotics. In addition, other medical conditions that frequently occur in the setting of antibiotic use such as surgery , the administration of muscle relaxants, and the presence of other debilitating diseases can contribute to impaired neuromuscular transmission. 44,45 Aminoglycosides
These are perhaps the most well-known antibiotic agents associated with neuromuscular transmission abnormalities, regardless of route of administration. 25,44 They appear to act via a wide range of pre-synaptic and post-synaptic mechanisms. Streptomycin can block the acetylcholine receptor and can also prevent the release of acetylcholine. 46,47 Neomycin is thought to act through ionic channels and block the acetylcholine receptors. 46 Kanamycin, clindamycin, and lincomycin are considered to act postsynaptically. 47 Gentamycin, tobramycin and amikacin are thought to affect the presynaptic release of acetylcholine. 47,48 The neuromuscular transmission defect is generally reversed by calcium and/or neostigmine. 47
Macrolides
Erythromycin may affect neuromuscular transmission by acting presynaptically, 48 and so may produce or worsen symptoms of myasthenia gravis in patients with pre-existing post-synaptic defects. 48 Exacerbations of myasthenia gravis have been reported with the use of telithromycin 49,50 and azithromycin. 51 Fluoroquinolones
This category consists of a large number of antibiotics, including ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, norfloxacin, ofloxacin, and trovafloxacin. These have been reported to cause neuromuscular transmission defects, 3,52 although the exact mechanisms are not known. Others
A few cases of worsening of myasthenic symptoms have been observed with the use of ampicillin 53 and other penicillins. Bacitracin may also worsen myasthenia, but the literature is confounded by the concomitant use of other antibiotics. 47 Polymyxins are considered to act both pre-and post-synaptically to produce or exacerbate myasthenia gravis. 25 Their action is reversed by the administration of diaminopyridine only. 47 Tetracycline and its analogues are reported to have weak neuromuscular blocking effects which usually are reversed by calcium, 47 and are thought to exacerbate myasthenia gravis. 23,25 There have been reports of worsening of myasthenia with the use of imipenem/cilastatin, 54 and with infusion of vancomycin. 55 The mechanism of action of these at the neuromuscular junction is not known.
We recommend avoiding the use of aminoglycosides in patients with known neuromuscular transmission defects. The other antibiotics discussed above should be used with caution in these patients.
Antiepileptics
Case reports and animal studies report unmasking and induction of myasthenic symptoms with the use of phenytoin, carbamazepine, trimethadione and gabapentin. 56,57,58,59 A variety of mechanisms have been identified. Long term phenytoin use may cause a depressed postsynaptic response to acetylcholine, inhibition at the level of calcium channels, and (at high concentration) an increase in the threshold of the muscle membrane. 60 Carbamazepine and trimethadione are thought to trigger an immune response with development of myasthenic symptoms. 58,61,62,63 Unmasking of myasthenia gravis is seen with the use of gabapentin, which is thought to bind to voltage gated calcium channels. 59 Remission is generally seen after withdrawal of these drugs. While none of these medications commonly unmask or induce myasthenia gravis, physicians should be aware of the possibility.
Miscellaneous Drugs That May Worsen Or Induce Myasthenia Gravis Chemotherapeutic Agents
The chemotherapeutic agents doxorubicin, etoposide, and cisplatin have been reported to exacerbate myasthenia gravis. 64,65 Respiratory and bulbar dysfunction have been noted within 24 hours of receiving these agents in combination, but cisplatin alone has been associated with respiratory worsening. The mechanism at the neuromuscular junction is unknown.
Interferon
The use of interferon alpha and beta for hepatitis C and multiple sclerosis has been associated with the development and exacerbation of a variety of myasthenic symptoms including ptosis, diplopia, dysarthria, and weakness, from one week to one year after beginning interferon treatment. 66,67,68,69,70 Elevated titers of acetylcholine receptor antibodies and decremental responses on repetitive nerve stimulation have been noted. 66 The mechanism appears to be autoantibody production, 66 and acetylcholine receptor antibodies have been found to persist even after discontinuation of interferon. The development of clinical findings of myasthenia gravis is not inevitable in patients undergoing interferon treatment. A patient with multiple sclerosis has been described who was seropositive but asymptomatic for myasthenia gravis, and who remained asymptomatic when treated with interferon. 71 It appears that interferon can be used in some individuals with myasthenia gravis, but the physician should be aware of the possibility of an exacerbation. Psychotropic medications
Lithium use can induce and unmask myasthenic symptoms. The mechanism is thought to be a decrease in the synthesis of acetylcholine and increased breakdown of acetylcholine receptors. 56,72 Lithium can also prolong neuromuscular blockade caused by neuromuscular blocking anesthetics. 25,44 Neuroleptics such as chlorpromazine and haloperidol, and tricyclic antidepressants such as amitriptyline and imipramine, are considered to have an adverse effect on neuromuscular transmission. 23,73 Caution should be exercised with the use of these psychotropic medications in myasthenic patients.
Antihelmintics
There is a case report of the antihelminthic drug pyrantel pamoate causing worsening of myasthenic symptoms. 74
Special Considerations -Anesthetics
There are some special considerations when using anesthetic agents in patients with myasthenia gravis. These vary depending on the class of agent. Neuromuscular blockers
Post-synaptic blockade is caused by depolarizing and nondepolarizing anesthetics. Patients with defects of neuromuscular transmission are particularly sensitive to nondepolarizing drugs 75 including atracurium, cisatracurium, doxacurium, gallamine, mivacurium, pancuronium, pipecuronium, rocuronium, tubocurarine, and vecuronium. Patients with fewer acetylcholine receptors such as myasthenics will show a rapid and marked neuromuscular blockade. 75,76 Another factor to consider is the inhibition of metabolism of some of these agents by pyridostigmine which may delay the recovery time. 75 In contrast, higher doses of depolarizing agents (succinylcholine, suxamethonium) are needed in myasthenic patients than in normal subjects. 77 Inhaled anesthetics
Agents such as sevoflurane, isoflurane, desflurane, halothane, enflurane, and nitrous oxide are used for intubation as well as maintenance of anesthesia. 78,79 Myasthenic patients require smaller amounts of these agents than normal, but the effects on neuromuscular transmission do not extend beyond the discontinuation of the agent, thus permitting rapid post-operative extubation of patients with myasthenia gravis. 79
Intravenous anesthetics
Drugs such as propafol 79 and opioids in therapeutic concentrations are considered to be safe for patients with defects of neuromuscular transmission. 75 Local anesthetics
Local anesthetics such as lidocaine, procaine, bupivacaine and esther anesthetics are thought to potentiate the effects of neuromuscular blocking agents. 25,75 However, the use of reduced dosages in myasthenic patients can decrease the chance of complications.
Figure 1
Conclusions
Many medications have been implicated as possibly worsening myasthenia gravis or inducing symptoms of myasthenia gravis in asymptomatic individuals. These are summarized in Table 1. Most of the data is in the form of case reports or very small series, making firm recommendations challenging. With the likely exception of aminoglycoside antibiotics, most other agents discussed in this review can be used in patients with myasthenia gravis if medically indicated, but should be employed with caution and close monitoring. In addition, anesthetic agents must be used with caution in these patients (Table 2). An awareness of the potential for exacerbating or inducing symptoms of myasthenia gravis should permit clinicians to engage in safer treatment of medical and surgical problems in these patients.