Exchange of a Conjugative Plasmid At Different Soil Moisture Levels Between Streptomyces Species Colonizing Artificial Soil Aggregates
B Bleakley, D Crawford
Keywords
conjugative plasmid, soil moisture, streptomyces
Citation
B Bleakley, D Crawford. Exchange of a Conjugative Plasmid At Different Soil Moisture Levels Between Streptomyces Species Colonizing Artificial Soil Aggregates. The Internet Journal of Microbiology. 2009 Volume 8 Number 2.
Abstract
Spores of a
Introduction
The ability to colonize and occupy solid substrates in soil, such as plant or animal remains, is vital to the growth and existence of soil microorganisms (Hissett and Gray, 1976). Genetically-engineered microorganisms (GEMs) which are released into the soil environment have to compete with native soil microorganisms during colonization and occupation of soil microhabitats. The outcome of such competitive colonization helps determine whether a GEM persists in the environment or dies off, influencing whether the GEM is able to manifest effects in the soil environment, or is able to transfer its DNA to native soil microorganisms (Stotzky and Babich, 1986).
Gene transfer events between soil prokaryotes have effects on phenomena including microbial evolution in general, and more specifically the spread of antibiotic resistance genes to microorganisms affecting human and animal health (reviewed by Thomas and Nielsen, 2005). Conjugation is one of the major types of gene transfer events, and is known to occur between many types of prokaryotes, including
The filamentous growth habit of some microorganisms (such as
The purpose of the present study was to see if larger, macroscopic “hot-spots” in the form of artificially molded, nutrient-amended soil aggregates would result in the same pattern of plasmid exchange as previously observed between a
Materials and Methods
Plate counts of streptomycetes in the soil incubations, to determine inoculum levels and final numbers, were performed with R2YE agar plates containing either thiostrepton to isolate
While the aggregates were still somewhat moist, they were placed into 12- ounce Ball jelly jars (13.4 cm high by 6.7 cm diameter). The jelly jars were closed with their usual screw-on metal lid. The opening of the lid was snugly closed with a number 12 rubber stopper. Each stopper had a hole drilled in its middle, which was snugly occupied by a permeable membrane closure (Kimble) allowing gas exchange to occur, but retarding water vapor loss from the soil. Aggregates were placed in jars to prevent moisture loss, until ready for autoclaving.
To sterilize and equilibrate aggregates at different moisture contents, aggregates were added to bulk soil which was adjusted to either 20%, 35% or 45% WHC. Aggregates (30 to 40 in number) were surrounded and covered with approximately 147.0 g ( oven dry basis) of bulk soil. The jars were sealed and autoclaved for 90 minutes daily for three successive days. After the final autoclaving, the aggregates were incubated at 30o C for one week to assure their equilibration with the surrounding soil. After equilibration, the 20% WHC aggregates were found to have a gravimetric moisture content of 13.7% wt/wt; the 35% WHC aggregates were at 16.9% wt/wt; and the 45% WHC aggregates were at 19.4% wt/wt.
Enough sterile 30.0 mM sodium phosphate buffer, pH 7.6, was aseptically added to each tube to give a 1:10 (wt/vol) dilution of soil. Tubes were vortexed for five minutes to evenly disperse the soil. Ten-fold dilutions were prepared from these for plate counts. Triplicate R2YE plates of each dilution were spread with 0.1 ml of soil suspension per plate. Sterile control tubes were plated as a check on sterility. The number of
Results
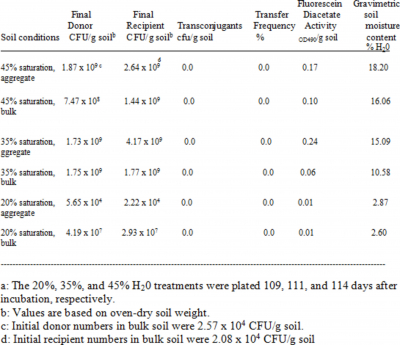
Macroscopic streptomycete growth was visible on most of the aggregates of inoculated treatments, except for those of the 20% WHC treatment in Table 1. Growth was not uniform over all the surface of an aggregate, but occurred in localized spots. No macroscopic growth was evident in bulk soil.
Although the jar assemblies did retard moisture loss, they did not completely stop it. Bulk and aggregate soil of all treatments lost water during the course of incubation ( Tables 1 and 2). The most extreme case occurred for the 20% WHC treatment of Table 1, although much of this moisture loss might have occurred during inoculation and manipulation of the bulk soil and aggregates. This jar assembly was probably the final one of three total 20% WHC jar assemblies prepared on day zero of inoculation.
Data for the 109-114 day-old, biparental cross incubations are shown in Table 1. The aggregates were initially sterile, so that streptomycete numbers on aggregates were largely due to colonization. The 20% WHC treatment is of interest because streptomycete numbers were lower than in the other treatments, probably due to the soil becoming too dry to allow continued streptomycete activity and colonization. This conclusion is also supported by the FDA results. No transconjugants were detected in any treatment for the jar assemblies analyzed in Table 1.
Transconjugants were detected on the aggregates for another 20% WHC jar assembly (Table 2), where the soil moisture was somewhat higher that the 20% treatment in Table 1. The spontaneous mutation rate of
Agarose gel electrophoresis on selected putative transconjugants verified that they possessed pIJ303 (data not shown).
Figure 1
Discussion
As in a previous study (Bleakley and Crawford, 1989), plasmid transfer between the donor and recipient used in this study was most frequent for nutrient-amended, low-moisture conditions. However, in the present study, the size of the nutrient “hot-spots” was much larger, in the form of artificial aggregates of soil mixed with nutrients. Results of the current study demonstrated that streptomycetes are able to colonize new habitats having little available moisture. For a number of silt loams in Idaho, the average field capacity is 24.2%, and the average permanent wilting point is 11.2% (McDole et al., 1974). Heterotrophic activity and plasmid exchange were detected in the present study at well below such moisture values. Streptomycetes are known to be more active in dry soil than very wet soil (Williams et al., 1972). The results reported here suggest that the soil moisture threshold for significant heterotrophic activity (for the strains and the soil employed) was between 2.87% and 4.22% wt/wt H20. In our previous study (Bleakley and Crawford, 1989) gravimetric soil moisture content as low as 9.3% allowed plasmid exchange to occur.
The CFU/g oven dried soil values varied most between bulk and aggregate for the 20% saturation incubation of 109 days incubation (Table1). Clearly the streptomycete numbers increased in the bulk soil, and colonization of aggregates occurred but streptomycete numbers on aggregates were the lowest for this incubation. No transconjugants were detected in this low moisture incubation. The aggregates may have dried out more in this incubation than other moisture treatments during preparation of the bulk soil/aggregate assemblies. The aggregates in the 20% saturation incubation of Table 2 did not dry as much, so that colonization and final numbers of streptomycetes were higher for this 126 day incubation. Transconjugants were detected in this 126 day low moisture treatment, and in no other treatment, and the transfer frequency (0.13%) was very comparable to that for an incubated moisture treatment amended with nutrients in the previous study (Bleakley and Crawford, 1989). Overall, numbers in bulk soil were approximately 108 CFU/g in this study for all moisture levels in Table 2, and were variable in the bulk soil of different moisture treatments in Table 1 but no lower than 107 CFU/g in the 20% saturation treatment. The present study suggests that soil moisture differences can lead to differences in streptomycete numbers and plasmid exchange frequency, in part due to difficulties in colonizing sterile aggregates across pore spaces separating bulk soil and aggregates. The lower CFU/g numbers in the current study compared to the previous work might also account for the lower number of transconjugants here compared to the 1989 study.
In the previous study (Bleakley and Crawford, 1989), incubations were for no longer than 69 days. In the present study incubations ranged from 109 to 126 days. It is interesting that FDA hydrolysis activity of nutrient amended aggregates at moisture contents of 35% and 45% saturation were still high and comparable to values from shorter incubations in the previous study. Perhaps the complex nature of the materials used to amend the aggregates helped promote extended heterotrophic microbial activity when moisture was adequate.
Unlike the previous study (Bleakley and Crawford, 1989), where transconjugants were detected in incubations of 40% and 60% WHC, in this study no transconjugants were detected in incubations at relatively high moisture contents (of 35% and 45% WHC). The nutrient-amended artificial aggregates did not promote plasmid exchange at these higher soil moisture contents to the degree that mixing the nutrient amendment as small particles into single-grained sieved soil did in the previous study (Bleakley and Crawford, 1989 ). The difference in physical nature of the organic amendments (small free particles mixed with single grained soil particles in the 1989 study; versus organic amendments molded and baked into larger soil aggregates in the present study) affected the frequency of plasmid exchange.
The current study allowed separation and analyses of nonamended bulk soil and nutrient-amended aggregate “hot-spots”. The spore inoculum added to bulk soil for each strain had to germinate, produce mycelia, and move from bulk soil to an aggregate in order to colonize aggregates. The filamentous growth habit of streptomycetes enables them to move across air-filled pored necks, such as likely existed to some degree between the bulk soil and aggregates. It appeared that at less than 2.87% H20 that water stress was too great to allow growth, activity, and/or movement of mycelia from bulk soil to aggregate.
Nutrient amendment was confirmed as being stimulatory to both plasmid exchange and FDA hydrolysis. It is interesting that exogenous nutrients were needed to stimulate plasmid transfer in this and the previous study (Bleakley and Crawford, 1989), since the autoclaved bulk soil had to contain major amounts of nutrients from dead soil microbial biomass. Since the spores of the strains employed in this study do not hydrolyze FDA (Bleakley and Crawford, 1989), the measured heterotrophic activity was due either to active mycelia or extracellular enzymes.
Colonization and plasmid exchange may be inter-related events for many streptomycetes in nature. Evidence suggests that
The results of this study are a further indication that soil moisture levels are a major environmental determinant of metabolic activity and plasmid exchange between soil streptomycetes. Studies of the molecular ecology of these filamentous soil microorganisms (reviewed by Marsh and Wellington, 1994) must always take soil moisture into consideration.
Acknowledgments
This work was supported by the Idaho Agricultural Experiment Station, and the South Dakota Agricultural Experiment Station. We thank David Hopwood for the gift of