Isolation and screening of different isolates of Aspergillus for amylases production
V Morya, D Yadav
Keywords
a. awamori, a. flavus, a. niger, a. tamarii, amylase, teak forest
Citation
V Morya, D Yadav. Isolation and screening of different isolates of Aspergillus for amylases production. The Internet Journal of Microbiology. 2008 Volume 7 Number 1.
Abstract
The diversity of
Introduction
Among a large number of non-pathogenic microorganisms capable of producing useful enzymes,
Materials And Methods
Microorganism
The indigenously isolated different isolates of
Chemicals
All analytical reagents and media components were purchased from Hi-Media (Mumbai, India), Merk BDH (Germany) and SISCO Research Laboratories Pvt. Ltd, (Mumbai, India).
Growth media
For isolation of
Media for fungal growth and spore production
Media used for fungal growth and spore production was standard Czapek’s agar containing 3.0 g sodium nitrate, 1.0 g dipotassium hydrogen phosphate, 0.5 g potassium chloride, 0.5 g magnesium sulfate, 0.001 g ferrous sulfate, 30.0 g sucrose and 15.0 g agar dissolved in 1.0 liter double distilled water, warmed and autoclaved after maintaining pH at 6.5(Raper and Fennell,1965).
Screening for amylase production
Preliminary screening for amylase production from
The amylase from different isolates were produced under submerged culture conditions using standard media comprising of Czapek solution with yeast extract, starch, peptone and thymine HCl. The final composition of the used media was 0.1 g yeast extract, 0.3 g peptone, 3.0 g NaNO3, 1.0 g KH2PO4, 0.5 g MgSO4, 0.01 g FeSO4, 10.0 g, soluble starch and 0.001 g Thymine HCl dissolved in 1.0 liter warmed double distilled water. The prepared media was aliquoted as 50 ml dispensed in 150 ml conical flasks prior to sterilization by autoclaving. The spore suspension with final concentration of 106 spore/ml was inoculated aseptically in triplicate sets of aliquoted and sterilized media. After 72 hrs of incubation at 300C under stagnant condition, the production of amylase was assayed by DNSA method (Miller, 1959).
Enzyme assay
Amylase assay was made by using a reaction mixture (1 ml of 2% starch solution mixed with 1.0 ml of 50 mM buffer, pH 6.5) and 0.1 ml of crude enzyme, incubated for 15 min. at 300C. After incubation 2 ml DNSA reagents was added and boiled for 10 min. and finally 1.0 ml of chilled 40% K-Na tartrate was added. The resulting colour due to reaction of DNSA and reducing sugar was measured at 540 nm wavelength (Miller 1959). One enzyme unit is equivalent to release of 1.0 µM maltose per unit time per unit volume.
Enzyme Kinetics
The amylase activity subjected to temperature ranging from 20 -100ºC was monitored by standard enzyme assay while thermostability of the enzyme was determined by incubating the crude enzyme preparation at temperatures ranging from 40-100ºC for 30 minute in a constant-temperature water bath (Lin,
Results
A total of 415 isolates of
The ten strains of
Table 1. Production chart of Amylases, Secreted by selected indigenously isolated
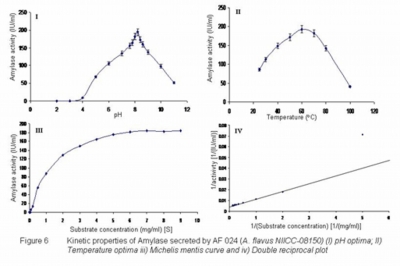
The amylases from different isolates were characterized for their basic properties and are shown in figure 2 to 16 for
The temperature optima of amylases produced by 14 isolate ranged from 30 to 60 while thermal tolerance of 50 to 80 was observed (Table 2). The amylase from
The Km values determined for amylase produced by all 14 isolates ranged from 0.6 to 2.85 mg/ml (Table 2) which is similar to what has been reported in literature (Kekos and Macris, 1983 and Kundu and Das 1970).
Discussion
Though the conditions of selected site for study is favorable for the growth of fungi and better niche for moderate mesophallic fungi but the results with screening of Amylases production shows the variability and potentiality among isolated
The results show that all
Acknowledgement
The authors are thankful to Head, Department of Biotechnology, D. D. U. Gorakhpur University, Gorakhpur, India for providing infrastructural facilities. One of Author V. K. Morya is thankful to CSIR, New Delhi for financial assistance as Senior Research Fellowship.