UV-B radiation and temperature stress causes variable growth response in Metarhizium anisopliae and Beauveria bassiana isolates
U Mustafa, G Kaur
Keywords
b. bassiana, germination, growth, m. anisopliae, temperature stress, uv-b radiation stress
Citation
U Mustafa, G Kaur. UV-B radiation and temperature stress causes variable growth response in Metarhizium anisopliae and Beauveria bassiana isolates. The Internet Journal of Microbiology. 2008 Volume 7 Number 1.
Abstract
The biocidal effects exerted through the activity of living organisms are practically more feasible and sustainable than the chemical cure. But one of the major threats for the bio-control agents is the on-field exposure to abiotic stresses. In this context, the present
Introduction
Commercial biological control involving entomopathogenic fungi in the present global scenario is a hi-tech venture both in terms of safety and sustenance. But the credibility of hi-fidelity management of insect pests by fungus is besotted upon attaining, maintaining and novel sustaining of such fungal strains, in the turmoil multitude of abiotic stresses (1). The continuance of viability and virulence of fungal inoculum (conidia) after field application is the pre-requisite threshold for their efficacy (2). Various isolates of
The solar radiation, which includes visible light, ultraviolet radiations, infrared rays and radio waves have been the dominant source in which all organisms evolved and adapted. In biological context, the UV radiations acclaim a special mention in terms of their impact on life (9). Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than soft X-rays. When considering the effect of UV radiations on organisms and the environment, the range of UV wavelength is often subdivided into UV-A or long wave or black light (400-315 nm), UV-B or medium wave (315-280 nm), and UV-C or short wave or germicidal (‹ 280 nm). UV-photons, in particular those belonging to the UV-B type, form covalent bonds between adjacent thymine bases resulting in thymine dimers. Thymine dimers do not base pair normally, which causes distortion of the DNA helix, stalled replication, gaps and misincorporation. These can lead to mutations and ultimately disrupt the normal functioning of the organism (10). Soil temperature is a major factor, which affects the success or failure in the establishment and production of fungal inoculum (11). The entomopathogenic fungi not only have to be tolerant to the soil temperature but also have to survive through thermoregulatory defense response of the host insect (12, 13). It has been demonstrated that stress temperature alters the vegetative growth among isolates of entomopathogenic fungi (14). Dry heat exposure causes DNA damage through base loss leading to depurination and this may cause mutation (15). Wet heat i.e. heat in conjunction with high humidity results in protein denaturation and membrane disorganization. It has been reported that
The entomopathogenic fungi are natural and cosmopolitan in occurrence. The purpose of this study has been to identify those naturally occurring entomopathogenic fungi, which are naturally resilient to abiotic stresses. With such an objective,
Materials and methods
Fungal cultures
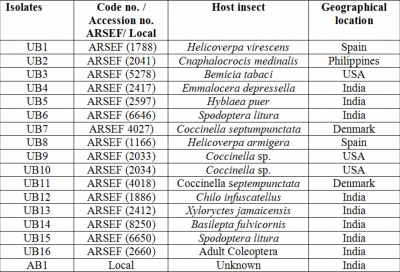
The different isolates of the fungus
Colony growth and sporulation (Temperature stress)
Seven day old cultures on SDA slants were used for preparing spore suspension in 0.02% Tween 80 solution at 1x106 spores/ ml. A 200μl of 106 spores were plated on SDA medium and were incubated for 3 days at 28C. At the end of 3 days, 5mm agar disc with mycelia was retrieved with the help of a cork borer and placed in middle of fresh SDA plates (5 replicates/ isolate were maintained) and incubated at 28C, 30C, 34C, 37C and 40C respectively. Radial growth were measured from 3rd day onwards till 8th day Radial growth rate (mmd-1) was calculated from the linear portions of the curves plotted from these values. The plates at 28C served as control. At the end of 8th day, 5mm agar discs were randomly taken with the help of a cork borer. The discs were placed in 10ml of 0.02% (v/v) Tween 80 solution and vortexed to suspend the spores. Spore concentration was determined using a Neubauer Haemocytometer.
Germination (Temperature stress)
Agar slide technique was used for studying the rate of germination. Petri plates were lined with blotting paper discs and 2 glass slides were placed in each of the plates and autoclaved. A 1ml SDA medium was evenly spread on each of the glass slides using micropipette. Conidial suspension was prepared from seven day old cultures with concentrations maintained at 106 conidia/ ml. Approximately 100µl of 106 spores/ml of fungal isolates were spread on the SDA coated slides. The slides were placed back in the Petri plates and the blotting paper discs were moistened with sterilized water. The Petri plates were kept for incubation at 28C, 30C, 34C, 37C and 40C (2 replicates/ isolate/ treatment were maintained). The slides were observed under compound microscope (40X) for germination, every 2 hours, starting from 4th hour after inoculation. A conidium was considered to be germinated when a distinct germ tube projected from it, and was at least twice the diameter of the conidium (17). Approximately 300 conidia were scored per replicate for each of the treatments and the rate of germination determined.
Germination (UV-B radiation stress)
A stock solution of 106 spores/ ml was prepared in a viol and vortexed and divided into 1ml each in 5 polystyrene tubes. The control-experiment tubes were wrapped in aluminum foil (0 hour exposure as the aluminum foil prevented UV-B penetration) and placed together with the test treatment tubes on UV-Trans illuminator, which served as the source of UV-B radiations (320 nm) and gave the desired exposure. Treatments (exposure duration) were 1, 2, 3 and 4 hours exposure to UV-B radiations. Petri plates were lined with blotting paper discs and 2 glass slides were placed in each of the plates and autoclaved. A 1ml SDA medium was evenly spread on each of the glass slides. Approximately 100µl of 106 spores/ml of fungal isolates, which were UV-B treated at different exposure durations, were spread on the SDA coated slides. The slides were placed back in the Petri plates and the blotting paper discs were moistened with sterilized water. The Petri plates were kept for incubation at 28C in dark (2 replicates/ isolate/ treatment were maintained). Observations were taken from 4th hour onwards till 24th hour, and repeated at every 4-hour interval. The slides were observed under compound microscope (40X) for germination. A conidium was considered germinated when a distinct germ tube projected from it. On an average about 300 conidia were scored/ replicate/ treatments and the rate of germination determined.
Statistical analysis
Variances in germination and growth counts among the different treatments and the sample time (days/ hours) were analyzed using procedures for two factor analysis of variance (ANOVA). The data of percentage germination were arc sine percentage square root transformed before analysis.
Results
Colony growth and sporulation (Temperature stress)
The fourteen
The response of different
Values followed by the same lower case alphabets in the same column are statistically equivalent (P<0.05) according to the Newman-Keul’s multiple range test.
Values followed by the same lower case alphabets in the same column are statistically equivalent (P<0.05) according to the Newman-Keul’s multiple range test.
Germination (Temperature stress)
The effect of temperature on the
The
Values followed by the same lower case alphabets in the same column are statistically equivalent (P<0.05) according to the Newman-Keul’s multiple range test.
Values followed by the same lower case alphabets in the same column are statistically equivalent (P<0.05) according to the Newman-Keul’s multiple range test.
Germination (UV-B radiation stress)
The UV-B dependent germination in
The
Values followed by the same lower case alphabets in the same column are statistically equivalent (P<0.05) according to the Newman-Keul’s multiple range test.
Values followed by the same lower case alphabets in the same column are statistically equivalent (P<0.05) according to the Newman-Keul’s multiple range test.
Discussion
Abiotic stresses, notably extremes in temperature, photon irradiance, water stress and variable concentration of organic-inorganic solutes, frequently limit growth and pathogenic potential of entomopathogenic fungi (18). In addition, more than one abiotic stress can occur at one time. Furthermore, one abiotic stress can decrease an organism’s ability to resist a second stress or vice-versa (8). Great variations in germination, growth and sporulation parameters was observed as isolate response to UV-B radiation and temperature stress, as anticipated from previous reports (19, 4) Certain isolates were appreciably robust in response to these abiotic stresses. Probably the physiology of tolerant isolates is more suited towards abiotic stresses and is thus indicative of their complex genetic base. The tolerant isolates exhibited variation in the phenotypic states depending on the stress stimuli and this capacity is supposed to be adaptive. Phenotypic plasticity exhibited by the entomopathogens was either a reversible or an irreversible plastic response, depending upon the reversion or persistence of the stress stimuli (20, 21). In the present study, it was observed that all
Abiotic stress tolerance is a polygenically determined attribute. Resilience to abiotic stress by inherent polygenic mechanisms is disadvantageous in terms of a rather complex genetic manipulation strategy for strain improvement. Consequently
Studies on UV-B tolerance suggest that with increasing exposure time, the rate of conidial germination declines (5). Fargues et al., 1996 (25) observed that conidia of
Acknowledgements
We acknowledge the financial support provided by Department of Science and technology (Project No. SR/ FT/ L-44). We also thank Dr. Richard A. Humber (USDA-ARS) for providing the fungal cultures.