Antimicrobial Activity of Eucalyptus major and Eucalyptus baileyana Methanolic Extracts
I Cock
Keywords
antibacterial activity, australian plants, medicinal plants, methanol extracts
Citation
I Cock. Antimicrobial Activity of Eucalyptus major and Eucalyptus baileyana Methanolic Extracts. The Internet Journal of Microbiology. 2008 Volume 6 Number 1.
Abstract
The antimicrobial activity of methanolic extracts of
Introduction
Plants produce a wide variety of compounds which in addition to giving them characteristic pigment, odour and flavour characteristics, may also have antimicrobial properties (Cowan, 1999). For thousands of years, traditional plant derived medicines have been used in most parts of the world and their use in fighting microbial disease is becoming the focus of intense study (Bhavnani and Ballow, 2000; Chariandy
Figure 1
Australian
The use of essential oils for the testing of antimicrobial activity is not without problems. The relative insolubility of many of the oil components retard their diffusion through agar gels in agar dilution or disc diffusion studies. Many studies have utilised solubilising agents (eg. Tween 80) to aid oil component diffusion, resulting in variable results (Griffin
Materials and Methods
Plant Collection and Extraction
The extracts investigated in this study have been described previously (Cock, 2008). Briefly,
Test Microorganisms
All media was supplied by Oxoid Ltd. All microbial strains were obtained from Tarita Morais, Griffith University. Stock cultures of
Evaluation of Antimicrobial Activity
Antimicrobial activity of each plant extract and was determined using a modified Kirby-Bauer (Bauer
The extracts were tested using 5 mm sterilised filter paper discs. Discs were impregnated with 10 µl of the test sample, allowed to dry and placed onto inoculated plates. The plates were allowed to stand at 4 oC for 2 hours before incubation with the test microbial agents. Plates inoculated with
Minimum Inhibitory Concentration (MIC) Determination
The minimum inhibitory concentration (MIC) of the plant extracts was determined by the disc diffusion method across a range of doses. The plant extracts were diluted in deionised water across a concentration range of 5 mg/ml to 0.1 mg/ml. Discs were impregnated with 10 µl of the test dilutions, allowed to dry and placed onto inoculated plates. The assay was performed as outlined above and the lowest concentration at which no zone of inhibition was observed was recorded as the MIC.
Bacterial Growth Time Course Assay
3 ml of bacterial cultures (
Results
Numbers indicate the mean diameters of inhibition of triplicate experiments ± standard deviation. – indicates no growth inhibition. Chl indicates chloramphenicol (10 µg) was used as the positive control. Amp indicates ampicillin (2 µg) was used as the positive control. Cip indicates ciprafloxicin (2.5 µg) was used as the positive control. Nys indicates nystatin nystatin discs (100 µg) was used as the positive control.
Both Gram-positive and Gram-negative bacteria were inhibited by
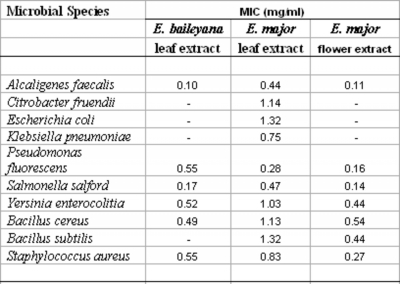
The relative level of antibacterial activity was evaluated by determining the MIC values for each extract against the bacteria which were shown to be susceptible by disc diffusion assays. MICs were evaluated in the current studies by disc diffusion across a range of concentrations. This has previously been determined to be a valid method of MIC determination as MIC values determined by disc diffusion correlate well with those determined by broth dilution assays (Gaudreau
Figure 3
Numbers indicate the mean MIC values of at least least triplicate determinations.
– indicates no growth inhibition.
The antibacterial activity of the
Figure 4
Discussion
The current study reports on the broad spectrum antimicrobial activity of two
Interestingly,
In summary, these studies confirm and extend the previously reported antibacterial activities of
Aknowledgements
Financial support for this work was provided by the School of Biomolecular and Physical Sciences, Griffith University, Australia.