Maternal And Perinatal Outcome In Pregnancy With Epilepsy
P Goel, L Devi, P Saha, N Takkar, A Huria, D Dua
Keywords
anti-convulsants, epilepsy, neonatal outcome, obstetric outcome, pregnancy
Citation
P Goel, L Devi, P Saha, N Takkar, A Huria, D Dua. Maternal And Perinatal Outcome In Pregnancy With Epilepsy. The Internet Journal of Gynecology and Obstetrics. 2005 Volume 5 Number 2.
Abstract
There was no statistically significant difference in the pregnancy related complications such as pregnancy induced hypertension(PIH), eclampsia, abruptio placentae, placenta previa, anaemia and gestational diabetes mellitus(GDM) in both the groups. Mean period of gestation (37.0 vs 37.7 weeks) was similar in both the groups. Rate of caesarian section was also similar in both the groups. Prematurity was observed in 21.6% cases and 17.6% in controls (p = 0.58). Fetal outcomes (APGARS score, birth weight,still births, neonatal death and congenital malformation) were also similar in the two groups. Seventy percent of the patient in study group were on monotherapy. Fourteen patients had seizure during pregnancy, but there were no maternal complications.
Introduction
Epilepsy is the most commonly encountered neurological disorder in Obstetrics after migraine. Incidence of seizure disorder in pregnancy is estimated to be 0.3 - 0.5% of all births.[1] Pregnancy with epilepsy is considered high risk mainly due to teratogenic potential of antiepileptic drugs and increased risk of pregnancy and neonatal complications i.e. hypertension, preeclampsia, antepartum hemorrhage, cesarean delivery, still births, neonatal deaths, intrauterine growth retardation and preterm delivery compared with general obstetric population. However, several studies have also been published that demonstrate no significant increase in these complications or intervention in pregnancy with epilepsy.[2,3,4] A few studies have focused on the effect of pregnancy on epilepsy and a possible worsening of epilepsy during pregnancy, but there are studies which do not show increase in seizure frequency during pregnancy and puerperium.[5,6,7]
The aim of this study was to determine the obstetric and neonatal outcome in epileptic women with pregnancy and the effect of pregnancy on epilepsy in a tertiary care referral hospital and to compare the outcome with women with no epilepsy who delivered in the same institute during the same time period.
Materials and Methods
A retrospective analysis was done of the records of all women with epilepsy who delivered at our hospital from January 1, 2000 to December 31, 2004 (n=37, study group). For each case three to four of age and parity matched healthy gravidas delivering at the same time were taken as controls (n=136,control group). Details of type, duration, frequency of seizures and drugs used for epilepsy were studied. Obstetric and neonatal outcome were compared in the two groups to know the effect of epilepsy on pregnancy. The following definitions were used:
Small for gestational age: All neonates with weight less than 1 standard deviation from the mean birth weight for the gestational age.
Low birth weight babies: Neonates with birth weight less than 2.5 kgs.
Statistical analysis was done using student t-test and chi square test. The statistical package SISS was used for analysis.
Results
Total of 15682 deliveries took place in the study period (January 1, 2000 to December 31, 2004) of which 37 women had epilepsy complicating pregnancy (0.23%).
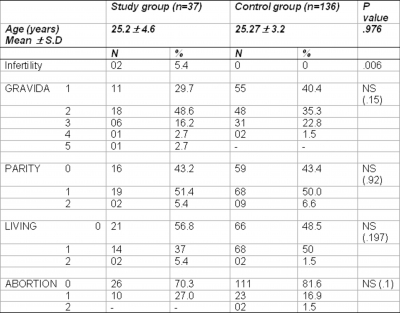
Demographic profile (Table 1)
There was no statistical significant difference between the two groups in number of living issues, parity and abortions. Mean age was similar in two groups (25.2 4.6 years and 25.2 3.2 years) in study and control group respectively. Infertility was reported in two patients in the study group but none among the control group (p=0.006).
Figure 1
Disease characteristic (Table II)
Out of 37 patients no details about the disease was available in three cases so these cases have been excluded from this analysis. twenty-three patients had generalized tonic clonic seizures, two had focal seizure and in nine patients nature of seizure was not mentioned in the records. duration of epilepsy was less than 10 years in 25 patients and 11 to 15 years in four patients and more than 15 years in five patients.
Fourteen patients had seizure during pregnancy (41%) and out of these three patients had first onset of epilepsy during pregnancy. Six patients with seizure during pregnancy had epilepsy of less than five years duration and most of the patients were not taking any anti-epileptic drug. Fifteen patients were on carbamazepine, five on phenytoin, two on phenobarbitone, two on sodium valproate, two patients were on ayurvedic treatment and seven were not on any antiepileptic drugs. One patient was on polytherapy taking combination of carbamazepine, sodium valproate and clonazepam. First trimester exposure was present in 24 cases. None of the patient experienced status epilepticus.
Delivery characteristics and maternal outcome (Table III)
There was no statistically significant difference in pregnancy complications like pregnancy induced hypertension, eclampsia, placenta praevia, abruptio placenta, anaemia, gestational diabetes mellitus in the study and the control group. Mean period of gestation at delivery was similar in both the groups (37.00 2.13 weeks) in study group and (37.65 1.92 weeks) in the control group. Induction of labour was higher (18.9%) in the study group as compared to the control group (7.4%) but this was not statistically significant. Rate of vaginal delivery and caesarian section was also statistically similar in both the groups.
Perinatal outcome (Table IV)
Mean period of gestation was similar in both the groups. Preterm babies were more in the study group (21.6%) than the control group (17.6%), although the difference was not statistically significant. Mean birth weight was similar in both the groups (2.68 0.51 kgs in the study group) and(2.71 0.53 kgs in the control group). There was no significant difference in Apgar score and proportions of neonates with low birth weight (< 2500 gm) in both the groups. Still births and neonatal deaths were also statistically similar in both the groups. There were no congenital malformations noted in the study group in their current pregnancies. In the previous obstetric history obtained, there were two preterm babies with hydrocephalous in one patient and both the babies died after birth; those pregnancies were unsupervised. Another patient had history of undergoing second trimester abortion due to hydrocephalus. Both the patients were on carbamezepine.
Discussion
Recent epidemiological studies report a prevalence rate of epilepsy to be 6.8 per 1000 population. It is one of the most common preexisting neurological disorders encountered by the obstetricians and affects approximately 1 in 200 pregnancies.[8] In our study the prevalence of epilepsy was 23 per 1000 births (0.23%).
Few population based studies of birth rate among epilepsy patients have been published. In most previous studies, fertility has been lower among epilepsy patients[9] than in the rest of the population, however conflicting results have also been reported. The reasons stated for low fertility are multifactorial such as decreased libido, social, genetic factors and also due to the side effects of drugs (anovulation). In our study, two patients in the study group had history of infertility compared to none in the control group. As the number is very small, definite conclusions cannot be drawn.
There are conflicting reports on the effect of pregnancy on epilepsy. Knight and Rhind[5] found that 45% of women with epilepsy had an increased frequency of seizures, 5% experienced a decrease and 50% had no change. A review of the literature revealed increase in seizure frequency from 23% to 75% amongst pregnant women who had epilepsy.[10] Although a recent study[7] have shown no increase in seizure frequency during pregnancy and perpeurium. The various reasons for an increase in seizure rate during pregnancy are due to changes in antiepileptic drug pharmacokinetics, sleep deprivation and decreased patient's compliance, which may be secondary to nausea and vomiting. We couldn't study the change in seizure frequency, as these details were not available, although 41% of patients had seizures during pregnancy in the study group. Trauma during seizures may result in placental abruption, preterm labour or premature rupture of membranes but none of our patient had these complications as a result of seizure.
Although there are variations within the published literature, many large-scale studies have shown that there appears to be 6.8% chance of birth defects in the infant born to woman taking antiepileptic drugs (AED). This represents a risk which is 2-3 times than that of the general population.[11] The risk is more with polytherapy as compared to montherapy.[12, 13] But there are few studies which do not show any increase in the rate of major congenital malformations (MCM's)[4] although they had majority of the patients on monotherapy. Evidence does not show that epilepsy per se is associated with a major increase in the risk of MCM. MCM's associated with AED's are congenital heart disease, neural tube defects, orofacial defects and urogenital defects. In a recent study the rate of MCM's was 2.4% in women with epilepsy not taking AED's, 3.4% on monotherapy, 6.5% on polytherapy. In the same study monotherapy associated major malformations rate was 2.3% for carbamezipine, 2.1% for lamotrigine and 5.9% for sodium valproate.[12] Valproic acid is the only AED for which a dose dependency has been confirmed in several studies, the increase in risk of MCM's compared with other AED's is especially evident at doses above 800-1000 mg/day.[12] In our study 24 patients (70%) were on monotherapy, 2 patients were on Ayurvedic medicine, one patient on polytherapy and 7 patients were not on any AED's. In the present study only two patients receiving carbamezapine had history of MCM's in three pregnancies (neural tube defects), however the current pregnancies supervised by us did not have any major MCM in the entire group. Data about the newer drugs and their effect is scanty as we have limited clinical reports and MCM's associated with lamotrigine, gabapentin and oxcarbazepine are not greater than the older AED's.[13] A recent review of literature of use of oxicarbazepine during pregnancy showed that there is no increase rate of malformations in newborns of women exposed to oxicarbazepine as compared to newborns in the general population.[14] I.Q among children of mothers with seizure disorder is low compared with controls. Infant born to mother with a seizure disorder of unknown cause are four times likely to develop idiopathic epilepsy. Epilepsy in father does not increase risk of developing a seizure disorder in children.[11] Folic acid has been shown to decrease the incidence of neural tube defects in women on AED's. All women on AED's should be prescribed folic acid in the dose of 5 mg/day which should be started preconceptionally and continued throughout pregnancy.[12]
Studies evaluating the effect of epilepsy on pregnancy are few. Some has shown an increase in pregnancy complications including hypertension, pre ecalmpsia, antepartum hemorrhage and cesarean delivery. There is also documentation of increased adverse neonatal outcome such as still births, neonatal deaths, premature deliveries, intrauterine growth retardation and lower neonatal APGAR scores.[2, 15] But some studies show no significant increase in obstetric and neonatal complication in women with epilepsy.[4, 16] In our study also there was no significant difference in the pregnancy complications (PIH, antepartum hemorrhage, anemia) between the two groups. Rate of cesarean section was also similar in the two groups. There was no difference in the neonatal outcome measure in the two groups (still births, small for gestational age, low birth weight babies, AGARS score)
Neonatal hemorrhage due to decreased Vitamin K
Conclusion
Our study showed that women with epilepsy can have uneventful pregnancies and can be assured of a good feto-maternal outcome in spite of approximately 41% having seizures during pregnancy. The effect of pregnancy on epilepsy could not be studied in detail because of unavailability of detailed data as this study being retrospective. As Indian literature is scanty about this medical disorder of pregnancy good large scale prospective studies are needed. Ideally women who have epilepsy during reproductive years must have effective preconceptional counseling. They should have planned pregnancies and should be told about the lowered efficacy of hormonal contraception. Patient should be controlled preferably with monotherapy and with the lowest possible dose. Folic acid should be given preconceptionally in the dose of 5 mg / day. She should be told the importance of continuation of antiepileptic drug and the drug levels should be measured in each trimester. Prenatal screening can detect major malformations. Vitamin K to be given 10 mg/day orally during the last month of pregnancy followed by Injection. Vitamin K 1 mg intramuscular to the baby. The various antepartum, intrapartum and neonatal complications can be significantly decreased by an inter-disciplinary cooperation between a neurologist, pediatrician and obstetrician.
Correspondence to
Dr P.K.SAHA House No. 1223 Sector 32B, Chandigarh – 160 032.INDIA Tel. No.: (0172) 2645262 e.mail: pklekha@rediffmail.com