Recall Bias can be a Threat to Retrospective and Prospective Research Designs
E Hassan
Keywords
bias, information, prevention, prospective, recall, retrospective
Citation
E Hassan. Recall Bias can be a Threat to Retrospective and Prospective Research Designs. The Internet Journal of Epidemiology. 2005 Volume 3 Number 2.
Abstract
Recall bias represents a major threat to the internal validity of studies using self-reported data. It arises with the tendency of subjects to report past events in a manner that is different between the two study groups. This pattern of recall errors can lead to differential misclassification of the related variable among study subjects with a subsequent distortion of measure of association in any direction from the null, depending on the magnitude and direction of the bias. Although recall bias has largely been viewed as a common concern in case-control studies, it also has been documented as an issue in some prospective cohort and randomized controlled trial designs. The aim of this paper is to address recall bias in selective studies employing retrospective and prospective designs and present some key methodological strategies to consider in analytic research using reported data in order to avoid or minimize recall bias.
Introduction
Bias is defined as deviation of results or inferences from the truth, or processes leading to such deviation1. It is the ultimate consequence of introducing systematic errors at any stage of investigation2. The term “bias” is sometimes referred to the lack of internal validity which is of central importance in epidemiologic research3. Among the several classifications of biases in the literature is the classification by Kleinbaum et al., who classified biases into three main classes: selection bias, information bias, and confounding4. Unlike confounding bias, selection and information bias cannot be corrected or controlled for after the completion of a study1. Therefore, it is critical during the planning stage of research to address the possible sources of these two biases and consider expedient strategies to avoid or at least minimize them.
Recall bias is a classic form of information bias1. It represents a major threat to the internal validity and credibility of studies using self-reported data5. According to Sackett's catalog of biases in analytic research, recall bias can be introduced in the data collection stage of investigation6. It arises when there is intentional or unintentional differential recall (and thus reporting) of information about the exposure or outcome of an association by subjects in one group compared to the other. This differential recall can lead to differential misclassification of the study subjects with regards to the exposure or outcome variable1. Recall bias of sufficient magnitude can depart the estimated measure of effect size either towards or away form the null, depending on the proportions of subjects misclassified. The risk estimate is biased away from the null if more cases incorrectly report being exposed or more exposed individuals incorrectly report developing a disease in case-control and prospective cohort studies respectively7.
Recall of information depends entirely on memory which can often be imperfect and thereby unreliable8. People usually find it difficult to remember or accurately retrieve incidents that happened in the past because memory traces in humans are not but poor versions of the original percept9. Research tells us that 20% of critical details of a recognized event are irretrievable after one year from its occurrence and 50% are irretrievable after 5 years10. Several mental processes contribute to this characteristic of humans' memory that often threatens the validity of self-reported data in analytic research: some details of an event may go unnoticed by the brain and thus never be stored in memory; memory tends to distort perception in systematic ways; repeated retrieval of already stored events may add new information as facts and thus events are re-stored in the brain in an altered fashion11. Given this complex non-dependable process of storing incidents, it has been concluded that the accuracy of recall in humans significantly depends on the time interval between the event and the time of its assessment: the longer the interval, the higher the probability of incorrect recalls12.
In general, recall bias can highly be expected in studies using reported data if one or more of the following conditions exist: the disease/event under investigation is significant or critical such as cancer or congenital malformation ; a specific exposure is preconceived by the patient as a risk factor of a high burden disease such as attributing increasing incidence of leukemia in a geographic area to electromagnetic fields produced by a nearby power lines; a scientifically ill-established association is made public by the media such as publicizing the ill-evident linkage between artificial light and risk of breast cancer; or the exposure under investigation is socially undesirable such as reporting of illicit drugs intake 12,13,14.
Although recall bias has largely been viewed as a constant major concern in case-control studies, it has also been documented as an issue in specific conditions of prospective cohort and clinical trial designs. The objectives of this paper are: to address recall bias in retrospective and prospective research designs and present key methodological strategies to consider in the design of research using reported data in order to avoid or minimize recall bias.
Recall Bias And Case-Control Designs
Participants in case-control studies mainly rely on their memory to identify what in the past might have caused their current disease which is most often of long latency. Because human memory is frequently imprecise, recall bias (According to Grimes and Schulz, 2002)1 is commonly believed to be “pervasive in case-control studies”. The presence of disease is presumed to act as a stimulus that affects both the patient's perception of the causes and his search for possible exposure to a hypothesized risk factor3. Therefore, the recall of remote exposures in case-control studies is commonly presumed to be differential among study subjects depending on their disease status15. Data, even about irrelevant exposures, are often remembered better by cases or/and underreported by controls16 . This
Logically, if recall of past events is unreliable if reported by subjects in case-control studies, then recall bias is more likely to be greater if information on past exposures is collected from a proxy18. This contention is supported by the conclusions of many case-control studies about the unreliability of responses from proxy respondents. For example, the evidence provided by two studies using proxy responses for two different associations: the use of herbicide 2, 4-dichlorophenoxyacetic acid and risk of non-Hodgkin's lymphoma; exposure to hazardous waste and risk of unfavorable respiratory health outcomes, was negated when the cases responded for themselves19, 20.
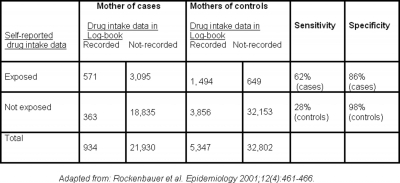
Recall bias has often been cited in case-control studies on congenital malformations or cancers in infants17. As noted previously, parents of children with serious congenital malformation have the incentive to recall all possible past events that could have caused the disease; whereas parents of healthy children lack such motivation. This is clearly demonstrated in the study by Rockenbauer and associates, 2001 21 which found that reported-data on drug intake during pregnancy by mothers interviewed few months after birth showed evidence of recall bias when compared to drug intake data recorded in a log-book by obstetricians during pregnancy. The sensitivity of exposure reporting was higher for cases than for controls. That means the proportion of truly exposed mothers correctly classified in the study was higher in cases than in controls, indicating better recall by mothers of cases. Furthermore, the noticed lower specificity of self-reported exposure for cases than controls indicates overreporting of the exposure by mothers of cases: the proportion of truly unexposed mothers correctly classified in the study was lower in cases than controls (Table 1). It is interesting to note that the timing of drug intake in this study was reported slightly closer to the time of interview for cases than for controls.
Figure 2
On the other hand, another group of investigators studying the same association have reported that recall bias might not be a major concern in case-control studies using parent-reported data as it has often been perceived. This argument received a substantial support from the results of a recent review of empirical studies that assessed the validity of parental reporting in case-control studies on different childhood diseases (leukemia, autistic disease, and sudden infant death syndrome) by using either adequate or gold standard data, such as medical records 22. The authors asserted in their review that a considerable number of 100 evaluated variables on past exposures suffered from inaccuracies in the reported related information equally by parents of both case and control subjects. Because nondifferential recall errors nearly always tend to depart the odds ratio towards the null value, they cannot account for the positive finding of a research and thus they are insignificant3. However, it is important to note that this rule may not hold and a bias away from the null can occur in nondifferential misclassification if the exposure variable has more than two categories23. Only a few of the evaluated variables in the review showed evidence of recall bias with a subsequent insignificant differential misclassification. Nevertheless, investigators of case-control studies using parental-reporting are constantly encouraged to consider use a proxy source of reported data if possible to evaluate whether differential reporting by study group has occurred5,22.
Advocating for the precautionary principle, results from case-control studies in general should be interpreted with caution because the pattern of recall bias frequently encountered in such design tends to inflate the estimated risk attributed to the exposure under investigation and this could potentially yield spurious association.
Recall Bias and Prospective Cohort Design
In prospective cohort studies using self-reported data, exposure data are collected before the occurrence of study outcome. Accordingly, prospective cohort design has been largely perceived as an effective strategy to avoid exposure recall bias that is frequently inherited in retrospective designs24, 25. However, it has been argued that differential recall of exposure is possible in prospective cohort studies if exposure variable is transient, with short induction period and repeatedly measured over time through self-report: e.g. episodes of anger or stress26. In this circumstance, there is opportunity for outcome onset to precede exposure self-report. This phenomenon is more likely to occur if the exposed individual has prior knowledge about the possible outcomes of an exposure. The empirical study by Kip et al26addressed recall bias in a prospective cohort study of the association between recurrent ocular herpes simplex virus (HSV) disease and systematic infection and psychological stress as putative risk factors. Findings from this study indicate that self-reported exposure data collected on or after the onset of the disease are more likely to be overreported (recalled better) when compared to the same data collected before the onset of the disease (a standard data collection process in the protocol of any prospective cohort study). This differential reporting can be explained by the concept of rumination bias: people with a disease tend to think harder about their prior exposures than disease free people6.
Recall bias and Randomized Controlled Trials
Randomized controlled trials (RCTs) with subjective outcomes may also be contaminated by recall bias if patients enrolled in the trial were not blinded to their treatment allocation. A participants' knowledge about what they receive may influence their reports of related effects, particularly if the outcome data are reported long enough after the fact. The study by Harnack and colleagues27 provided an excellent example of recall bias in this specific condition of RCT design. The authors examined intervention-related bias in recalling and reporting of food intake in a population of American Indian children enrolled in several elementary schools randomly assigned to a diet intervention program or a control condition. When the authors compared self-reported data of 24-hour dietary intake collected the next day with direct observation of children while eating their school lunches as an objective measure of the outcome, they found that girls in the intervention schools systematically underreported their dietary intake relative to girls in the control schools. This trend was not found in boys. The authors attributed the differential reporting of food intake by intervention condition to social desirability bias which might be greater among girls in the intervention schools, where healthy eating is emphasized in the classroom curriculum. Recall bias may be most marked in RCTs if people who collect self-reported outcome data are not blinded to treatment allocations1.
Approaches to Minimize Recall Bias
Irrespective of study design, the first step in the process of avoiding any type of bias is the proper definition and articulation of the research question. Consequently, this step will lead to a number of questions that need to be adequately addressed by the investigator during the planning stage of research: what kind of information are required to answer this question in the study in terms of exposure, outcome, and possible confounders; what is the most appropriate method to collect these information; and how to achieve comparable accuracy of data collection between the study groups. According to previous research, the accuracy of recall generally depends on: the degree of required detail bout the exposure or outcome28; interviewing techniques and the quality of questionnaire; and to some extent the personal characteristics7, 12. All of which are important factors to consider in the planning for recall bias elimination.
In case-control designs
Despite the fact that recall bias is a major limitation of case-control studies, a number of methodological strategies documented in the literature can minimize recall bias:
-
Using nested case-control design in which reported data on exposures are collected at baseline and throughout a cohort study, if feasible
29 . -
Choosing newly diagnosed cases because remote diagnosis may lead to reporting of newly adopted behaviors as a consequence of the disease
12 . -
Choosing appropriate control group: Finding the perfect control group in case-control studies can be challenging. Many epidemiologists advocate for using patients with a disease not related to the exposure as valid surrogates for population controls
6 ,30 ,31 . This suggestion is based on the assumption that diseased controls are similar to the cases in their concern about the possible causes of their disease, thus the comparable accuracy principle between the two groups is not violated32 . A limitation of this strategy is the possibility of introducing other type of biases such as sampling bias which may occur when the diseased controls have exposures different from those of the general population12 . Another limitation is the possibility of choosing controls with a disease that has unknown (unexplored) relationship with the exposure. Another group of investigators advocate for using healthy individuals as controls because they proved to be an adequate reference group in some empirical studies33 . To avoid this debate, some researchers have suggested the use of two control groups in the same study (if possible): a group of healthy individuals and another of diseased controls. Although the latter suggestion may seem more reassuring, it can give rise to confusion if the results were different between the two control groups32 . The widely accepted strategy in the scientific community is to choose the most appropriate control group within the study context32 . -
Using standardized data collection protocols: information about exposure should be collected in the same way and at similar timing for cases and controls
1 . -
Using a well-structured and validated instrument for exposure assessment. The instrument should probe detailed questions about the exposure to help the participants report accurate recalls: the number of exposure events, duration of each event, ect
20 . -
Applying the instrument at similar timing in both study groups
34 . -
Giving the participants enough time before answering to reflect and think through a sequence of events in their life history
10 ,35 . -
Blinding the study subjects to the study hypothesis and the specific factors being studied. As an example, questions about exposure of interest can be asked among a long list of questions covering other potential exposures
17 . -
Blinding the data collector/interviewer to the outcome status of subjects and the study hypothesis
1 . -
Verification of exposure reported-data by using a reference criterion (e.g. medical records) or another source of reported-data (e.g. data from a spouse or a twin-sibling)
5 . -
Conducting a subgroup analysis by the subject knowledge of the purported association to determine if bias exists in the conducted study
36 .
In Prospective designs
-
Using standardized data collection protocols: information about outcome should be collected in the same way and at similar timing for exposed and unexposed.
-
Blinding the participants to study hypothesis and RCTs to treatment allocation
-
Blinding the observer/data collector to the study hypothesis, exposure-status of the participants in cohort or treatment allocation in RCTs.
-
Verification of the self-reported data about the outcome via proxy sources, such as direct observation or use of biological markers.
Conclusion
Research including reported data about past experiences will always be threatened by the limitations of the individual's memory and the influence of disease/exposure status on the recalling process in humans. Case-control studies are the most subjected design in analytic research to recall bias. However, differential recall is also possible in prospective cohort studies if exposure status is transient, must be periodically recalled and reported, and ascertainment occurs after symptom onset. Empirical studies suggest that recall bias can be a concern even in randomized controlled trials including subjective outcomes if measurements are collected after a period of time from the incidence of outcomes. To avoid or minimize recall bias while designing similar studies in the future, investigators should consider a number of methodological approaches including: use of standardized well structured questionnaire; blinding subjects and data collectors to the study hypothesis; and using proxy sources of reported data if available.