ST-Segment Elevation Myocardial Infarction; Part I Diagnosis: A Case Study Approach
L Ikpeama, S Ruppert
Keywords
acute coronary syndrome, acute myocardial infarction, myocardial infarction, st elevation myocardial infarction
Citation
L Ikpeama, S Ruppert. ST-Segment Elevation Myocardial Infarction; Part I Diagnosis: A Case Study Approach. The Internet Journal of Advanced Nursing Practice. 2012 Volume 11 Number 2.
Abstract
Acute myocardial infarction (AMI) is a common disease diagnosis in industrialized society, with approximately 650,000 patients with new AMI and 450,000 experiencing a recurrent AMI each year in the United States. This diagnosis continues to carry a significant mortality rate, particularly in elderly males. This first of two articles presents a case study of an elderly patient who, after coronary intervention, develops a ST elevation myocardial infarction. The significance of interpreting the physical and diagnostic data in diagnosing the event is discussed along with the related pathophysiology. Treatment will be discussed in a subsequent article.
Introduction
Acute myocardial infarction (AMI) is a common disease diagnosis in industrialized society, with approximately 650,000 patients with new AMI and 450,000 experiencing a recurrent AMI each year in the United States (US).1 AMI is a major health burden, with estimated direct and indirect cost at $165.4 billion per year.1 The estimated number of years of life lost due to an AMI is approximately 15 years.1 Although mortality rate has declined by approximately 30%, 1 in 25 patients who survive admission for AMI die in the first year after event. AMI predominantly affects middle-aged to older men, but incidence increases in women at menopause.2 Elderly patients who are over 75 years of age have a four-fold mortality compared to younger patients.2 AMI is also gaining grounds in developing countries as a problematic disease burden.3
This case study discusses an elderly man who had previously undergone a bare metal stent (BMS) for a diseased left anterior descending (LAD) coronary artery. Because of past history of thrombotic events, the patient was previously worked up for inherited thrombophilia, and found positive for heterozygous Factor V Leiden (FVL) mutation and was placed on life-long anticoagulation with warfarin. Prior to stent placement, the patient’s long-term warfarin was discontinued and enoxaparin was started. Post-procedure, enoxaparin was continued while awaiting a therapeutic international normalized ratio (INR) on re-initiation of warfarin. Two days before anticipated discharge, the patient once again experienced acute excruciating chest pain; same quality as on initial admission, but now unrelieved by nitroglycerin. Electrocardiogram (ECG) showed ST segment elevation. This case highlights the importance of early recognition of ST elevation myocardial infarction (STEMI) and immediate activation of the cardiology STEMI team. Prompt, early, and effective treatment of the patient experiencing myocardial infarction (MI) supports early goal achievement that leads to improved outcomes. The case was complicated by difficult primary percutaneous coronary intervention (PCI) and gastrointestinal bleeding while on antiplatelet and anticoagulation immediately after PCI for prevention of stent stenosis. Challenging treatment decisions regarding complications encountered during the patient’s management made this case complex.
Encounter Day 1 (10 days post intervention)
History of Present Illness
The patient was an 85 year-old white male with a past medical history listed below. The patient was admitted to cardiology service with complaints of 1-2 weeks exertional chest pain. On admission, cardiac enzymes were negative, and initial ECG was negative for ST/T wave changes. The ECG on admission showed normal sinus rhythm (NSR) with old inferior infarct shown as q-waves on leads III and AVF. A right axis deviation (RAD) was present that may be indicative of an old anterior-lateral MI. The patient underwent a nuclear medicine stress test, and was found to have inducible myocardial ischemia involving the left ascending diagonal (LAD) vascular territory with preserved left ventricular ejection fraction (LVEF) of 60%. A left cardiac catheterization showed a severely stenotic proximal LAD and 95% lesion. Successful deployment of Vision 2.75X18mm BMS, with 0% residual stenosis was achieved. Hospital discharge was delayed for achievement of therapeutic INR, and the patient’s inability to self-administer enoxaparin injections at home.
Ten days later, the patient complained of severe chest pain lasting 10 minutes. He described the pain as “crushing and escalating”, associated with dyspnea, nausea, and radiation to left jaw and arm. The rapid response team was called. (Information below and in Tables 1-4 were at the time of the first encounter).
Past Medical History
Social History
Family History
Discussion of Testing
Electrocardiography
Electrocardiogram (ECG) is the single most important test that aids diagnosis of STEMI, and should be performed and shown to an experienced provider within 10 minutes of a patient’s complaint of chest pain whether inpatient or arrival to the ED. The ECG is at the center of decision pathway for treating patients that present with this symptom. If initial ECG does not show STEMI but the patient’s symptoms persist with a high clinical suspicion of STEMI, serial ECGs every five to ten minutes or continuous 12-lead ECG is recommended to detect potential development of ST elevation.3 (Further discussions on ECG will under the Diagnostic Review section).
Cardiac Biomarkers
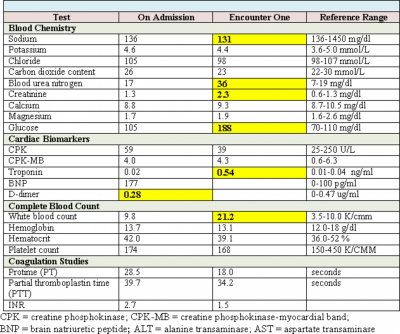
Cardiac biomarkers such as CPK, CK-MB, and troponin are proteins released from infarcted heart muscle after any damage to the myocardium as seen in STEMI and NSTEMI. These substances become detectable in serum when cardiac lymphatics become overwhelmed and unable to clear the amount being spilled into the venous circulation.2 These biomarkers can be useful in the diagnosis of myocardial damage, and provide supportive non-invasive evidence for reperfusion therapy; however pending lab results should not delay therapy for patients with ST elevation on ECG and symptoms of STEMI.3 Due to their high sensitivity and specificity for myocardial damage, cardiac troponins are preferred for diagnosis of myocardial damage and infarction. Cardiac troponins typically rise within 3-6 hours of a cardiac event and therefore may not typically be elevated during initial work-up for a patient that presents with chest pain suspicious for an MI, and may persist for up to 14 days.3 This patient’s troponin level was not elevated on initial admission but significantly elevated at 0.54 ng/ml at the onset of chest pain. This finding leads to the suspicion that a previous episode of unstable angina or vague epigastric discomfort may have been a MI. CK-MB is the second option if cardiac troponin assays are not available, and has the advantage of diagnosing patients who present early with symptoms. This biomarker appears rapidly in the blood long before troponins are detected, but returns to normal within 24-48 hours. CK-MB, however, is found in the blood of healthy people with any skeletal muscle damage, and is not as specific as cardiac troponin in detecting myocardial damage.3 This biomarker can therefore be used to estimate the time of injury in patients with elevated troponins, as well as detect re-infarction during a treatment course, 1 and is very useful in assessing reperfusion following treatment.3
Other Laboratory Examinations
These organizations cautioned, however, that pending laboratory test results should not delay treatment, especially reperfusion therapy for a patient with STEMI. Other laboratory tests may be indicated in screening for or monitoring co-morbid conditions such as this patient had.
Complete Blood Count
A complete blood count is warranted in order to follow trends while a patient is on anticoagulation therapy. The patient and CBC need to be monitored for evidence of obvious bleeding, anemia and thrombocytopenia. With anticipation of double and triple anti-platelet agents during reperfusion therapy, the provider needs to evaluate baseline platelet count level.5
Basic Metabolic Panel
This laboratory panel evaluates electrolytes as well as the status of acid-base balance, the kidneys, and the liver. If a patient has been receiving diuretics, electrolyte replacement may be needed based on results. Monitoring of kidney function is warranted when a patient is undergoing reperfusion therapy where large amounts of dye will be administered.6 Additional therapeutic interventions may be warranted if contrast nephropathy is anticipated in patients with marginal renal function.
Coagulation Studies
These tests (PT/PTT/INR) are used to determine clotting time values for an individual. Each individual test can be used to evaluate the effectiveness of anticoagulation therapy, and to guide dosage modification. PT/INR is used to evaluate the therapeutic effects of warfarin, while PTT evaluates therapeutic effect of unfractionated heparin. These coagulation studies also can give an indication of underlying clotting abnormalities.7 In this case, the patient was currently on enoxaparin and warfarin, and effectiveness of the warfarin needed to be assessed.
Brain Natriuretic Peptide (BNP)
BNP is a brain hormone that is released in the heart with increased levels in heart failure, with plasma levels increased in patients with symptomatic and asymptomatic left ventricular dysfunction. BNP can also be elevated in some patients with coronary heart disease, valvular heart disease, constrictive pericarditis, pulmonary hypertension, and sepsis.8 For this patient, the rationale is unclear why BNP was part of an initial diagnostic evaluation, but may have been presumably due to complaints of associated shortness of breath on initial presentation to the emergency department (ED).
D-dimer
D-dimer is a simple peripheral blood test with a high negative-predictive value for DVT when used properly. This test provides a valuable screening tool for providers to rule out the presence of deep vein thrombosis or pulmonary embolus (PE) and reduce the need for more time-consuming and expensive testing. Testing for plasma D-dimer in the ambulatory care or ED setting has emerged as an excellent non-invasive triage test for patients with suspected DVT or PE.9, 10 In populations like the elderly, pregnant women, and those with recurrent DVT or PE, d-dimer results are less reliable.9 The value can also be elevated in normal physiologic states as well as other disease states, and should not be used alone in diagnostic evaluation of PE or DVT. 9, 10 Because of prior history of PE and DVT, a D-dimer level may have been deemed necessary as part of initial work-up for this patient.
Diagnostic Review
Electrocardiography
Electrocardiography is a fast, non-invasive, and accessible bedside screening tool that guides emergency treatment strategies for patients that present with chest pain. Because of decreased blood supply, a 12-lead ECG may show ST segment elevation for patients with acute myocardial infarction.11 ACC and AHA recommend that a 12-lead ECG be performed and read by an experienced practitioner within ten minutes of presentation to the emergency department by all patients with chest pain or symptoms suggestive of a STEMI. The initial ECG can shed light on patients with important predictors of mortality such as a left bundle branch block (LBBB), and anterior injury/infarct location, and can guide choice of reperfusion therapy.3 This patient’s ECG on admission and at the time of the event 10 days later can be seen in Figures 1 and 4 below. Figure 2 is ECG on encounter 1 showing diffuse ST elevation (leads I, AVL, V3, V4, V5, V6,) indicating anterior-lateral injury, left axis deviation (negative in lead II), right bundle branch block (split R wave in lead V1). Note pathological Q waves in these anterior-lateral leads with marked repolarization abnormalities indicating injury to the myocardium. Mortality also increases with the number of ECG leads showing ST elevation.3
Chest x-ray
Kushner et al.,4 writing for ACC/AHA, recommend a high-quality portable chest x-ray as one imaging study that should be used to differentiate a STEMI from aortic dissection in a subset of patients whose diagnosis is unclear. Aortic dissection appears as a widened mediastinum on chest film. However, an imaging study should not delay implementation of reperfusion if a definitive diagnosis of STEMI exists.3 Chest radiography is also indicated when a relevant history or physical examination calls for further investigation of pulmonary symptoms such as cough, shortness of breath, suspicion of pneumonia, lung congestion, and mass or any other finding that may be significant in clinical patient care.4 The case study patient complained of chest pain associated with shortness of breath, and physical exam also supported the need to get an imaging of his cardiac silhouette and lung parenchyma (shown in Figures 3 and 4).
Impression:
The heart is upper normal in size. Moderate pleural thickening is demonstrated at the inferior part of the chest on both sides and at both apices. The appearance is similar 2 months ago. There is no significant interval change. Presence of any new air space consolidation is not suspected. The pulmonary vascularity is within range of normal
Impression:
Probable bilateral small pleural effusions, with associated basilar sub-segmental atelectasis. Early pneumonia in the right lower lobe is not excluded. Posterior-anterior and lateral views of the chest are recommended when feasible.
Differential Diagnoses
Working Diagnosis: ST segment elevated myocardial infarction
Pathophysiology
Myocardial infarction occurs when a coronary artery suddenly becomes partially or completely blocked by a blood clot, causing at least some of the heart muscle being supplied by that artery to become injured or permanently damaged due to an imbalance of oxygen supply and oxygen demand.2,12 Signs and symptoms that suggest MI include: chest pain (pressure, squeezing, pain in the center of the chest), discomfort in one or both arms, back, neck, jaw, or stomach, shortness of breath, nausea and vomiting, weakness, fatigue, and diaphoresis.3 Elderly patient are less likely to experience chest discomfort and are more likely to present with shortness of breath, nausea, and syncope.3 In patients with diabetes, pain recognition may be altered due to autonomic neuropathy. These patients may present instead with dyspnea, nausea/vomiting, diaphoresis and/or fatigue.3 Women may also present without the chest pressure. Atypical symptoms need to be evaluated in these special populations or in individuals at high risk for coronary heart disease (CHD).
Atherosclerotic plaques have long been implicated in the pathology of heart disease. ST elevation myocardial infarction (STEMI) is not usually precipitated by slow-building high-grade stenosis of the coronary artery that may cause complete occlusion of the vessel, rather by non-obstructive plaques that are made with abundant macrophages and inflammatory cells. These plaques are lipid laden, and are referred to as vulnerable and high-risk plaques that are easily disrupted. Once disrupted or injured, exposure of substances occurs that promotes platelet aggregation, activation and adhesions, as well as thrombin generation and formation. The formed thrombus can completely occlude the coronary artery, and without sufficient collateral circulation, myocardial ischemia and subsequent infarction occurs within 15 minutes of myocardial oxygen deprivation.3 In STEMI, the coronary artery previously affected by atherosclerosis is abruptly occluded by a thrombus at the site of vascular injury and completely blocks off oxygen and blood supply to all the heart muscle being supplied by the affected artery causing death to the heart muscle.
STEMI in very rare cases may be due to coronary artery occlusion caused by coronary emboli, congenital abnormalities, coronary spasm, and other systemic inflammatory diseases, but in most cases occurs when the surface of an atherosclerotic plaque becomes disrupted, exposing the intima to an inflammatory process that favors thrombogenesis. A mural thrombus forms at the site of plaque disruption, and the involved coronary artery becomes occluded.1, 2 (See Figure 3) This injury process is produced or facilitated by factors such as smoking, hypertension, and lipid accumulation.
Acute coronary syndrome (ACS) is an umbrella term which encompasses a spectrum of myocardial ischemia including: unstable angina (USA), myocardial infarction without ST segment elevation (NSTEMI), and STEMI.1,2 USA is non-specific chest pain syndrome that may indicate ischemia with or without ischemic changes seen on ECG, but without abnormal cardiac biomarkers.13 STEMI shows persistent ST-segment elevation in two or more contiguous leads on ECG with elevated cardiac markers, while NSTEMI does not show elevation of the ST-segment, but also has elevated cardiac biomarkers. Non-specific ischemic changes like ST depression or T wave inversion may also occur in NSTEMI and USA. The percentage of patients with ACS who develop STEMI is 2%.1The previously classification of Q-wave and non–Q-wave MI for ACS recently has been abandoned as not all STEMIs evolve into a Q-wave MI, and a NSTEMI may develop a Q-wave MI. Recent classification is based on the initial or subsequent ECG findings: those with ST-elevation (STEMI) and those without ST elevation (NSTEMI), but with enzyme evidence of myocardial damage. The current classification coincides with current treatment strategies because patients presenting with ST elevation can benefit from immediate reperfusion therapy.1, 2
Major Risk Factors of Coronary Heart Disease
Coronary heart disease is one components of the metabolic syndrome, and a major risk factor for MI.14 Primary care providers have been charged with identifying and assisting patients with modifiable risks of CHD to reduce such risk factors. Some of the risk factors of CHD that can result in MI are smoking, family history of CHD, hyperlipidemia, hypertension, abdominal obesity, and diabetes mellitus.
Patients with two or more risk factors are at increased ten-year risk and can benefit greatly from primary prevention. Patients with established CHD also benefit from secondary prevention of the disease.3 Several health promotion and prevention strategies may help in the reduction of fatality from STEMI. These include:
Killip’s Classification of Patients with STEMI
Risk stratification of patients with STEMI through physical assessment can be achieved by using the Killip’s classification of STEMI (Table 5) to predict clinical outcomes and mortality based on the degree of heart failure following acute STEMI.1 This classification stratifies STEMI patients into four groups based on the degree of heart failure (HF). Killip's Class I is STEMI patients with no evidence of heart failure who have a 6% mortality risk. Class II is those with mild HF as evidenced by rales, S3 heart sound, and congestion evident on chest imaging. This class of patients has a 17% mortality rate. Class III is patients with STEMI who have developed subsequent pulmonary edema, and have a 38% mortality rate. STEMI patients in cardiogenic shock are classified under Killip’s class IV, with an 81% mortality risk. Early assessment and risk stratification of patients with acute STEMI enables prediction of outcomes.1
{image:10}
Conclusion
AMI is a disease with high mortality. When AMI manifests as STEMI, the presentation is a medical emergency and should be managed as such. The availability of effective, time-sensitive treatment for STEMI and the emphasis on affordable health care is the impetus for clinicians to ask the right questions, to obtain the right answers, and to institute appropriate interventions to maximize patient outcomes and reduce hospital lengths of stay. Prompt recognition of STEMI is crucial and begins with recognition of patients presenting with chest pain or signs/symptoms suggestive of ACS or STEMI as high-priority triage cases and avoidance of delays at first contact with the healthcare system. Confirmation of STEMI by ECG is immediately required as early management may be the difference between survival and death. In aiming for best outcomes, each institution must devise the most efficient guideline-based pathway to ensure rapid triage and management of patients. The approach involves a multi-disciplinary team effort that includes emergency room providers, the rapid response team, cardiologists, nurses, and support staff working to achieve the goals of STEMI treatment which include relieving ischemic pain, stabilizing patients, and administering beta-blocker, anti-platelet and appropriate reperfusion therapies. Comprehensive treatment of STEMI is presented in Part II of this article.
Acknowledgement
The authors thank Jane A. Anderson, PhD, RN, FNP-BC, Associate Director Stroke Center, Michael E. DeBakey Veterans Affairs Medical Center in Houston Texas for facilitating data collection for this article.