Myasthenia Gravis: Towards A Safer Anesthesia Technique. Clinical Experience And Review Of Literature.
M Elarief, E Ibrahim, P Magadi
Keywords
anesthesia, anticholinergic, muscle relaxant, myasthenia gravis, remifentanil, sevoflurane
Citation
M Elarief, E Ibrahim, P Magadi. Myasthenia Gravis: Towards A Safer Anesthesia Technique. Clinical Experience And Review Of Literature.. The Internet Journal of Anesthesiology. 2006 Volume 11 Number 2.
Abstract
Myasthenia gravis (MG) is a disease with many implications for the safe administration of anesthesia and involves considerable morbidity and mortality. Thymectomy is a common surgical procedure in patients with myasthenia gravis. We have here described an anesthesia technique involving continuation of preoperative anticholinesterase, use of non-paralyzing technique (i.e. avoidance of muscle relaxants), and use of ultra-short acting anesthetics. This technique was safe and effective in all of our 8 patients who underwent trans-sternal thymectomy. All our patients were extubated on table following administration of intravenous anticholinesterase and anticholinergic drugs. There was no postoperative morbidity or mortality. None of our patients needed ventilatory assistance in the postoperative period.
Introduction
Myasthenia gravis (MG) is an autoimmune disease characterized by weakness and fatigability of skeletal muscles, with improvement following rest. Myasthenia gravis is caused by autoantibodies to postsynaptic nicotinic acetylcholine receptors (anti-AChRs) at the neuromuscular junction, causing weakness of skeletal muscles. In addition to these autoantibodies, patients with thymoma-associated MG produce autoantibodies to a variety of neuromuscular antigens, including antibodies to the skeletal muscles calcium release channel and antibodies to cytoplasmic filamentous proteins. Some patients with thymoma- associated MG have an inflammatory myopathy of striated and cardiac muscles. Cardiac myositis may cause heart failure, cardiac arrhythmias, and sudden death. Neuromyotonia also can be associated.
The diagnosis of MG is suggested by clinical history and is confirmed by electromyography, anticholinesterase test or serological assay for acetylcholine receptor antibody. The incidence of (MG) is 50–142 cases per million population(1). The disease may be localized to specific muscle groups or it may be generalized. Most commonly, the eyelids and extra ocular muscles are involved. Bulbar involvement may be manifested as difficulty in chewing and swallowing. “Myasthenic crisis” refers to a rapid deterioration in neuromuscular function with respiratory compromise precipitated by ventilatory muscle insufficiency, weakness of upper airway musculature, or some combination of these two processes(2). Myasthenia Gravis may be associated with thymoma, other autoimmune disorders such as SLE, rheumatoid arthritis, pernicious anemia, thyrotoxicosis, or, rarely cardiomyopathy(2).
Treatment of MG may be medical or surgical, utilizing one of three approaches: anticholinesterases (medical), immune suppression (medical), or thymectomy (surgical). Pyridostigmine (Mestinon) in a dose of up to 120 mg p.o. every 3 hours is used because it is tolerated well orally, with few muscarinic side effects, and has a relatively long duration of action. Immune suppression is directed at preventing or attenuating the destruction of acetylcholine receptors at the motor end plate(3), using corticosteroids, azathioprine, cyclosporine or a combination of these. For patients with severe bulbar symptoms or respiratory compromise (myasthenic crisis), plasmapheresis is used. Immune globulin given intravenously has been used to treat myasthenic crises when plasmapheresis cannot be used.
Majority of patients with MG have thymic abnormalities: hyperplasia in 60 to 70 percent and thymoma in 10 to 12 percent(2). Thymectomy is the commonest surgery done for patients with Myasthenia Gravis. In general, MG centers have developed protocols for the care of MG patients and have a team of neurologists, surgeons, pulmonologists, intensivists and respiratory care specialists, nurses and anesthesiologists caring for MG patients undergoing thymectomy. The most frequently used procedure is the extended form of trans-sternal thymectomy. Its proponents believe that it gives the best assurance that in most instances the entire thymus is being removed and performed safely, and that it produces the best long term results.
Aim of the Study
Our aim is to provide a safe general anesthesia technique involving continuation of preoperative anticholinesterase, avoidance of muscle relaxants, use of short acting anesthetics, slow and cautious reintroduction of anticholinesterase after surgery, extubation on operating table and avoiding postoperative ventilation.
Patients And Methods
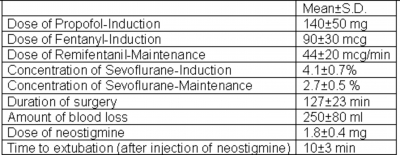
Eight patients underwent thymectomy from January 2003 to October 2005, who were not considered to be difficult intubation, did not have pre-existing pulmonary disease and were not in crisis or in need of preoperative ventilatory support were enrolled for the study. Preoperative evaluation included careful assessment of respiratory function and airway reflexes to predict the need for potential need for postoperative ventilation. We did not use sedatives to premedicate our patients. Anxiolysis was achieved by detailed and compassionate briefing about the procedure. We continued all the doses of medications preoperatively including anticholinesterase to ensure patients psychological support and to avoid aggravation of symptoms and muscle weakness. Patients on steroids were supplemented with a dose of methylprednisolone 100 mg i.v. before the induction of anesthesia in the operating room. Two peripheral venous lines and an arterial line were secured. Monitoring involved 5 lead ECG, invasive arterial pressure, pulseoximetry, nasopharyngeal temperature, end tidal CO2, arterial blood gas and electrolyte analysis, inspired and expired concentrations of oxygen and anesthetic agents and nerve stimulator with TOF mode of stimulation. After preoxygenation, anesthesia was induced with 2 micrograms/Kg of fentanyl, 1.5 - 2 mg/Kg of propofol . With the help of sevoflurane 2-4% in oxygen, mask ventilation was performed to facilitate laryngoscopy, the airway was secured with an appropriate size ET tube using a non-paralyzing technique (i.e. without the use of muscle relaxant). One dose of Cefotaxime, 2gm, was administered as the antibiotic. Anesthesia was maintained with sevoflurane 2 -3% in (50%oxygen - 50%air mixture) and remifentanil infusion titrated to effect. We avoided factors which can enhance neuromuscular blockade- hypothermia, hypokalemia, and aminoglycosides. Before closure of the wound paracetamol 1gm i.v.was given. At the end of the procedure, the anesthetic agents were discontinued and the patients received neostigmine, in a dose based on the preoperative pyridostigmine dose (2mg i.v. neostigmine is equivalent to 60mg of oral pyridostigmine) and glycopyrrolate 10micrograms/kg. Patients were extubated with adequate muscle power and respiratory pattern and a TOF ratio of > or equal to 0.8, sustained head lift for > or equal to 5sec., and they were able to generate a negative inspiratory force of >25 cm of H2O. They were monitored in the surgical intensive care unit for 12 hours in the postoperative period and analgesia given on demand.
The ease of intubation was graded by the same anesthesiologist as either excellent, good or poor based on 6 attributes-jaw relaxation, resistance to laryngoscopy, vocal cord position, vocal cord movement, limb movement and diaphragmatic activity. The overall intubating grade assigned to each patient was the lowest grade in any of the 6 attributes.
Results
All our 8 patients who underwent trans-sternal thymectomy using the above technique were extubated on operating table following anticholinesterase and anticholinergic. The ease of intubation was graded as “excellent” in 5 (62.5%) patients and as “good” in 3 (37.5%) patients. Time to extubation after injection of neostigmine was 10±3 min. There was no postoperative morbidity or mortality. None of our patients needed ventilatory assistance in the postoperative period.
Figure 6
Discussion
Preoperative Evaluation and Preparation of the MG patient includes review of the severity of the patient's disease and the treatment regimen. Specific attention should be paid to voluntary and respiratory muscle strength. The patient's ability to protect and maintain a patent airway postoperatively may be compromised if any bulbar involvement exists preoperatively. The ability to cough and clear secretions may be compromised as well. Respiratory muscle strength can be quantified by pulmonary function tests (negative inspiratory pressure and forced vital capacity). These tests may be necessary as a reference to determine the optimal conditions for extubation postoperatively as well as the need for postoperative mechanical ventilation (4,5).
Thymectomy is a surgical procedure commonly undergone by patients with MG. If a thymoma presents an anterior mediastinal mass, intrathoracic airway or vascular obstruction may occur upon the induction of anesthesia. Flow-volume loops may be indicated preoperatively.
Several general anesthetic techniques have been proposed, although none has been proven to be superior to the others. Some choose to omit anticholinesterase on the morning of surgery, to decrease the need for muscle relaxants(6), whereas others continue its use for psychological support of the patient. We preferred to continue the preoperative doses of anticholinesterase even on the morning of surgery. If the patient is poorly controlled, a course of plasmapheresis may be of benefit in the preoperative period(7). Such patients and patients with respiratory compromise were excluded from the present study. The steroid-dependent patient will require perioperative coverage. In the present study, patients on steroids were supplemented with a dose of methylprednisolone 100 mg i.v. before the induction of anesthesia in the operating room. Anxiolytic, sedative, and opioid premedications are rarely given to patients who may have little respiratory reserve. If the patient has primarily ocular symptoms, a small dose of benzodiazepine is acceptable(1). We did not offer preoperative sedative to our patients to avoid respiratory compromise.
Neuro muscular Blockers, The myasthenic patient is typically sensitive to nondepolarizing neuromuscular blockers. Sensitivity to nondepolarizing agents has been described in patients with minimal disease (i.e., ocular symptoms only)(8), in those in apparent remission(9), or those with subclinical undiagnosed myasthenia(10). Intermediate and short-acting nondepolarizing agents can be used with careful monitoring of neuromuscular transmission, preferably with electromyogram (EMG) or mechanomyogram (MMG), which measure the evoked electrical or mechanical responses following electrical stimulation of a peripheral motor nerve. Myasthenic patients are similarly sensitive to cisatracurium, as evidenced by a more rapid onset and more marked neuromuscular block compared with control patients(11). The presence of fade (T4/T1 < 0.9) in the preanesthetic period predicts decreased atracurium requirements in patients with MG(12). In our patients we did not use any muscle relaxants and we avoided factors which can enhance neuromuscular blockade- hypothermia, hypokalemia, and aminogycosides.
Inhaled Anesthetic a g e n t s , may cause muscle relaxation in normal patients(13). There may be some variability in the response among myasthenics(13,14). Sevoflurane at 2.5% (slightly greater than 1 MAC) depresses (EMG) responses, T1/ Tc by 47% and T4/T1 by 57%(15). Russel et. al. found sevoflurane was suitable as a sole anesthetic for a myasthenic undergoing sternal split thymectomy, implying that sevoflurane alone provided adequate muscle relaxation(16). Sevoflurane appears to depress neuromuscular transmission to the same degree as isoflurane, although in one myasthenic patient the sensitivity was much greater (>85% T1 suppression)(17). Also sevoflurane is rapidly eliminated at the end of surgery due to it's low blood solubility. Sevoflurane is probably superior to desflurane, due to its lower incidence of excitatory airway reflexes during inhalational induction. In our patients we achieved the relaxation required for intubation and surgery with sevoflurane.
Intravenous Anesthetic Agents: Propofol has the theoretic advantages of short duration of action without effect on neuromuscular transmission. Opioid analgesics in therapeutic concentrations do not appear to depress neuromuscular transmission in myasthenic muscle(18,19). However, central respiratory depression may be a problem with opioids. The introduction of short-acting opioids makes these drugs more titratable in the myasthenic. Remifentanil's short elimination half-life (9.5 minutes)(20) makes the drug appealing.
Interactions with other Drugs, Many commonly used drugs affect neuromuscular transmission to a small degree. In normal patients, this is usually of no clinical significance. In the myasthenic patient, upon emergence from anesthesia and surgery, the interactions of these drugs with residual anesthetic effect and the disease state of MG may be more significant. The drugs which are known to affect neuromuscular transmission in myasthenics and are preferably avoided are - aminoglycoside antibiotics and the polymyxins(21,22,23), beta adrenergic blockers (24,25), corticosteroids (23,26), procainamide (27), and phenytoin(28). We did not use any of these drugs in our patients except the supplemental dose of corticosteroids in patients who were already on steroids.
The decision as to whether to reverse residual neuromuscular blockade at the end of surgery is controversial. Some argue that the presence of anticholinesterases and antimuscarinics will confuse efforts to differentiate weakness due to inadequate neuromuscular transmission from cholinergic crisis in the recovery room. They prefer spontaneous recovery and extubation when the patient has demonstrated adequate parameters for extubation (i.e., head -lift, tongue protrusion). However, we were able to achieve adequate neuromuscular transmission and extubation on table by reinstituting intravenous anticholinergic(neostigmine) in a dose substituting the oral dose of pyridostigmine. 2mg i.v. neostigmine is equivalent to 60mg of oral pyridostigmine.
Total intravenous anesthesia (TIVA) for the management of myasthenics has been reported(29). In our study we have adopted a balanced anesthetic technique using short acting inhalational and intravenous anesthetic-analgesic agents but without using muscle-relaxant.
Postoperative Considerations: There have been several attempts to predict the need for postoperative ventilation (4,30,31). Based on the preoperative condition of the patient, the surgical procedure, and the residual anesthetic effects, a carefully planned extubation may be carried out in most patients. Adequate postoperative pain control, pulmonary toilet, and the avoidance of drugs that interfere with neuromuscular transmission will facilitate tracheal extubation. All patients with MG should be closely monitored postoperatively in the post anesthesia care unit or the surgical intensive care unit, where respiratory support can be immediately reinstituted. Weakness after surgery presents a special problem in MG patients. The differential diagnosis includes myasthenic crisis, residual effects of anesthetic drugs, nonanesthetic drugs interfering with neuromuscular transmission and cholinergic crisis. For these reasons, many clinicians prefer to avoid the use of muscle relaxants, or if they use muscle relaxants, allow the neuromuscular block to recover spontaneously, avoiding the use of anticholinesterase in the immediate postoperative period. Meticulous attention to pulmonary toilet is required, particularly since respiratory secretions may be increased by the cholinesterase inhibitors. In our patients we did not use any muscle relaxants and post-operatively we reintroduced anticholinesterases intravenously to obtain adequate muscle power and respiratory function. All our patients were extubated on table following anticholinesterase and anticholinergic. There was no postoperative morbidity or mortality. None of our patients needed ventilatory assistance in the postoperative period.
Conclusions
Myasthenia gravis is a disease with many implications for the safe administration of anesthesia. We have here described an anesthesia technique involving continuation of preoperative anticholinesterase, use of non-paralyzing technique (i.e. avoidance of muscle relaxants), use of ultra-short acting anesthetics and extubation on operating room table, in our patients who underwent thymectomy. This technique was safe and effective in all of our 8 patients who underwent thymectomy. A multicentric study involving a larger number of patients is needed to validate this technique.