Rosuvastatin: Just Another Statin?
A Bandari, M Davidson
Keywords
hmg co-a reductase inhibitors, ldl-c, primary prevention, rosuvastatin, secondary prevention, statins
Citation
A Bandari, M Davidson. Rosuvastatin: Just Another Statin?. The Internet Journal of Cardiology. 2003 Volume 2 Number 2.
Abstract
Convincing evidence exists supporting the major role HMG Co-A reductase inhibitors (statins) play in both primary and secondary prevention of cardiovascular diseases given, among other effects, their essential role in the reduction of LDL-C cholesterol . Recently revised guidelines recommend even more intense management of LDL-C cholesterol especially in moderate and high risk patients. Rosuvastatin, the newest statin, offers the most beneficial effects on the lipid profile and particularly, LDL-C. Its safety profile, both pre-approval and post-approval, at the current FDA-doses of 5-40mg is similar to other available statins. Thus, rosuvastatin is an extremely helpful addition to the lipid armamentarium when prescribed at the current FDA approved doses.
Introduction
The benefits of the 3-hydroxy -3-methylglutaryl coenzyme A reductase inhibitors (statins) for both primary and secondary prevention of cardiovascular disease have been clearly elucidated over the past decade in numerous trials beginning with 4S and culminating in the recently published PROVE-IT trial (1,2,3,4,5,6,7,8). The evidence supporting the use of statins has been so convincing that these compounds are now the most prescribed compounds on the market. Still, many patients on statin therapy are unable to reach NCEP ATP III goals and others who may benefit are not on therapy altogether (9,10). Furthermore, based on the most up to date evidence, recently published revisions in NCEP ATP III have been formulated (11). The updated guidelines, among other suggestions, recommend that in very high risk patients, a goal LDL of <70mg/dl is optimal. Thus, while many patients were not at goal previously, the new guidelines will further reduce the percentage of patients at their LDL goal.
The Options
As of today, six different statins are approved by the FDA for patient use: atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin. Rosuvastatin is the most recently approved of the statins and was introduced in August 2003. Since its introduction, this agent has heralded both great optimism and controversy. Rosuvastatin demonstrates the most pronounced dose-related reductions in LDL cholesterol of any available statin (12,13,14). Moreover, it has a highly favorable effect on HDL cholesterol values when compared to other statins (12). Still, the 100 deaths resulting from the use of cerivastatin and its subsequent withdrawal from the market has led to a fair amount of reluctance by the medical community to accept any new statins.
Rosuvastatin: Is It Superior To Other Statins?
Rosuvastatin , a synthetic statin, acts as an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA reductase), the principle enzyme involved in the rate-limiting step in cholesterol biosynthesis. Its half-life is roughly 19 hours, longer than any other statin on the market (15). Unlike many other statins, rosuvastatin has minimal affect on cytochrome P450. Thus, it has a much lower risk of interactions with many other commonly used pharmacological agents (16).
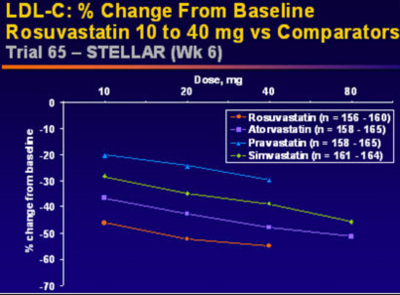
Rosuvastatin's effects on the entire lipid profile and particularly LDL-C are superior to any other statin on the market (12,13,14). In the STELLAR (Statin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin) Trial, 2431 hypercholesterolemic adults (LDL-C > 160 mg/dl and < 250 mg/dl; Triglycerides <400 mg/dl) were randomized to treatment with rosuvastatin 10, 20, 40, or 80 mg; atorvastatin 10, 20, 40 or 80mg; simvastatin 10, 20, 40, or 80mg, or pravastatin 10, 20, or 40 mg. Serum lipid compositions were then taken after 6 weeks of therapy. Across dose analysis, it was demonstrated that rosuvastatin 10-40 reduced LDL-C by a mean of 8.2% more than atorvastatin 10-40 mg, 26% more than pravastatin 10 to 40 mg, and 12-18% more than simvastatin 10-80mg (all with P<.001)(12). This data reiterated previously published dose to dose comparisons.
Rosuvastatin also offers superior effects on HDL cholesterol compared to others in its class. At 6 weeks of therapy in the STELLAR trial, rosuvatatin 10 to 40 mg demonstrated a +7.7% to +9.6% increase in HDL compared to a +2.1% to +6.8% increase with atorvastatin, pravastatin, and simvastatin at various doses. Furthermore, unlike atorvastatin, rosuvastatin displayed more pronounced increases in HDL with dose titration upwards. In addition, rosuvastatin faired better than all the statins studied with respect to total cholesterol and triglyceride reductions in this study. Most importanty, in STELLAR, more rosuvastatin patients reached NCEP ATP III and European LDL cholesterol goals than any of the other therapies (12).
Statin Safety: The Lesson's-Learned From Cerivastatin
While as a class, the FDA approved statins have been well tolerated with very few adverse events, cerivastatin is the exception and many lessons can be learned from its approval and subsequent discontinuation. Cerivastatin .4mg was approved in the US in May 1999 and cerivastatin .8mg was approved thirteen months later. Of all the statins, cerivastatin was unique given its extremely high potency and bioavailability. Its pre-approval myopathy rates were double the highest previously approved rate but given the safety of other statins, these rates were overlooked (17). Moreover, reports were beginning to surface regarding its life-threatening reactions with gemfibrozil which led to warnings on the coadministration included in package labels. Mainly, gemfibrozil as a CYP 2C8 inhibitor (18) and inhibitor of statin glucuronidation, causes marked increases in cerivastatin blood levels (19). Such coadministration leads to a 20 to 80 fold increase in the rate of rhabdomyolysis and ultimately led to over 100 deaths despite warnings by the manufacturer against using the drugs in combination (20). Cerivastatin was removed from the market in August of 2001
Rosuvastatin Safety After Cerivastatin
Given the heightened sensitivity to the adverse events of statins in the post-cerivastatin era, the approval of rosuvastatin by the FDA involved much more scrutiny than any previous approval of a statin. This necessitated the pre-approval database to include considerably more patients than any previously approved statin. Doses of 5mg to 80mg were evaluated. The myopathy rates in the 5mg to 40mg populations ranged from .1%-.2% of the over 16,000 patients evaluated. For the 80mg dose, the incidence of rhabdomyolysis was .4% which prompted the makers of rosuvastatin to discontinue the development of this dose for clinical use. No cases of rhabdomyolysis were observed at any of the other doses(21).
In addition to rhabdomyolysis, 12% of patients taking 80mg of rosuvastatin developed an increase of > 2+ urine dipstick proteinuria, a finding not previously seen with statin therapy (21). These findings were transient and not associated with a compromise in observed renal function. In the Stellar trial, two female participants developed acute renal failure although confounding variables were present in both cases (12). These latter events led to a delay in approval of rosuvastatin but ultimately, the FDA approved the drug at doses of 5 to 40 mg.
{image:9}
Since its introduction into the market in August 2003, the safety profile of rosuvastatin at the FDA-approved dosing range of 5-40mg is similar to the other statins available today. Even with such a profile, rosuvastatin has still received negative press in relation to its safety profile both in the medical and lay press. In
Earlier this year, a consumer's public health watch dog group strongly petitioned the FDA to withdraw its approval of rosuvastatin. The group, Public Citizen, included several cases of rhabdomyolysis as reasons for its protest (23). In particular, the group cited a case of a 39 year old woman who developed rhabdomyolysis on rosuvastatin. After careful analysis, however, it was determined that the etiology of the woman's rhabdomyolysis was an acute myocardial infarction. Rhabdomyolysis is a rare but well-documented adverse event of all statins and its incidence with rosuvastatin therapy at 5mg-40mg is comparible to the incidence with other approved statins according to the FDA Adverse Event Report database (24).
Rosuvastatin Dosing/Interactions
The currently recommended starting dose of rosuvastatin is 10 mg dose with titration to 20 mg only if necessary. Caution is advised when increasing the dose to 40mg. It is a class-wide phenomenon that increasing the dose of a statin results in a multiple-fold higher risk of developing myopathy. Thus, this recommendation exists across all statins and not just rosuvastatin. Furthermore, the incidence of myopathy is no higher with rosuvastatin than with other statins at the same dose and not one case of rhabdomyolysis was witnessed in over 16,000 patients taking rosuvastatin in the pre-approval data-base at the 5-40 mg doses (21).
{image:10}
While rosuvastatin is only minimally involved with the cytochrome P450 pathway and drug-drug interactions are few, extreme caution should be exercised with co-adminstration of rosuvastatin with warfarin, cyclosporine, and gemfibrozil. Rosuvastatin may cause a more pronounced increase in INR for patients on warfarin and thus, close monitoring of patients should be exercised when rosuvastatin is initially prescribed for patients taking warfarin. This caution should also be applied when doses are titrated until a fairly steady INR and warfarin dose is achieved (25).
Cyclosporine increases the dose of all statins including rosuvastatin by several fold. Thus, the only recommeded dose of rosuvastatin for patients on cyclosporine should be 5 mg. In patients with severe renal impairment, similar dosing modifications should be followed given that in such patients, rosuvastatin is partially renally excreted. Doses should not exceed 10 mg in these patients(25).
As with other statins, gemfibrozil does raise serum rosuvastatin concentrations (17). For patients taking gemfibrozil 600 mg bid, the Cmax and AUC of rosuvastatin increases by 2.2 and 1.9 fold respectively (23). Thus, we do not recommend the use of rosuvastatin and gemfibrozil together especially given the cerivastatin outcomes. If combination therapy is deemed necessary to achieve NCEP ATP III goals, the use of ezetimibe, fenofibrate, or colesevelam is a safer alternative depending on the clinical scenario. If gemfibrozil is to be used in combination with rosuvastatin, the manufacturer suggests that only the 5mg and 10mg doses are prescribed (25).
With the recently released guidelines recommending more intense reductions in LDL cholesterol levels, rosuvastatin should be seriously considered as the primary choice of therapy in high risk patients and those unable to reach their recommended LDL goals. In addition, given the fact that rosuvastatin has minimal interaction with the cytochrome P450 system, it also provides the added benefit of fewer drug to drug interactions than most other statins. Still, outcome data with rosuvastatin is not yet available given its relatively short time on the market with respect to other statins. Such data is currently being compiled. That withstanding, given the superiority of rosuvastatin in the modification of the entire lipid profile when compared to all other statins and its equivalent safety profile to other statins, rosuvastatin is an extremely helpful addition to the lipid armamentarium when prescribed at the current FDA approved doses.